Sea Water Desalination with Reverse Osmosis Systems
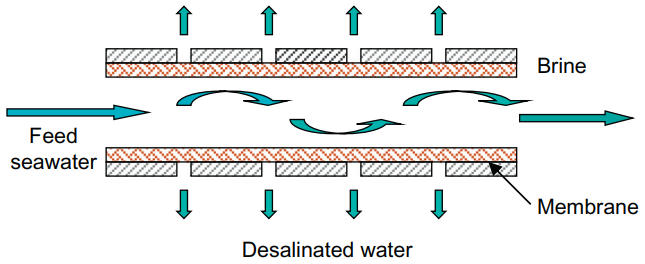
RO separates water from salted seawater by delivering mechanical energy to a semipermeable membrane in the form of pressure. The pressure in the salt solution is higher than the osmotic pressure, and its amount is determined by the salt content in the solution. RO is a single-phase technique that operates at low temperatures.
Longer membrane life and higher procedure performance were accomplished by extensive research on semipermeable membranes, and seawater reverse osmosis desalination (SWRO) joined the water market around the middle of 1973. Since then, RO has found a wide application for seawater desalination, and it is competitive with distillation technologies, which are presently employed in about half of all facilities across the world. Membrane modules come in a variety of shapes and sizes, including tubular, spiral wound, and hollow fibre.
The pre-treatment area is the biggest issue for RO plants in the Middle East and abroad. Many plants have been affected by seasonal organic blooms, intense biological activity, and turbidity, biofouling necessitates, membrane chemical cleaning and productivity loss. Maintaining the required filtrate silt density index (SDI) levels throughout the year has proven problematic.
Water quality parameters required especially in seawater, TDS, hardness, alkalinity, and organic scaling depending on the seawater involved reverse osmosis process related water quality parameters. Reverse Osmosis is a golden standard of seawater desalination plant. So, the most of desalination plant consists of many reverse Osmosis.
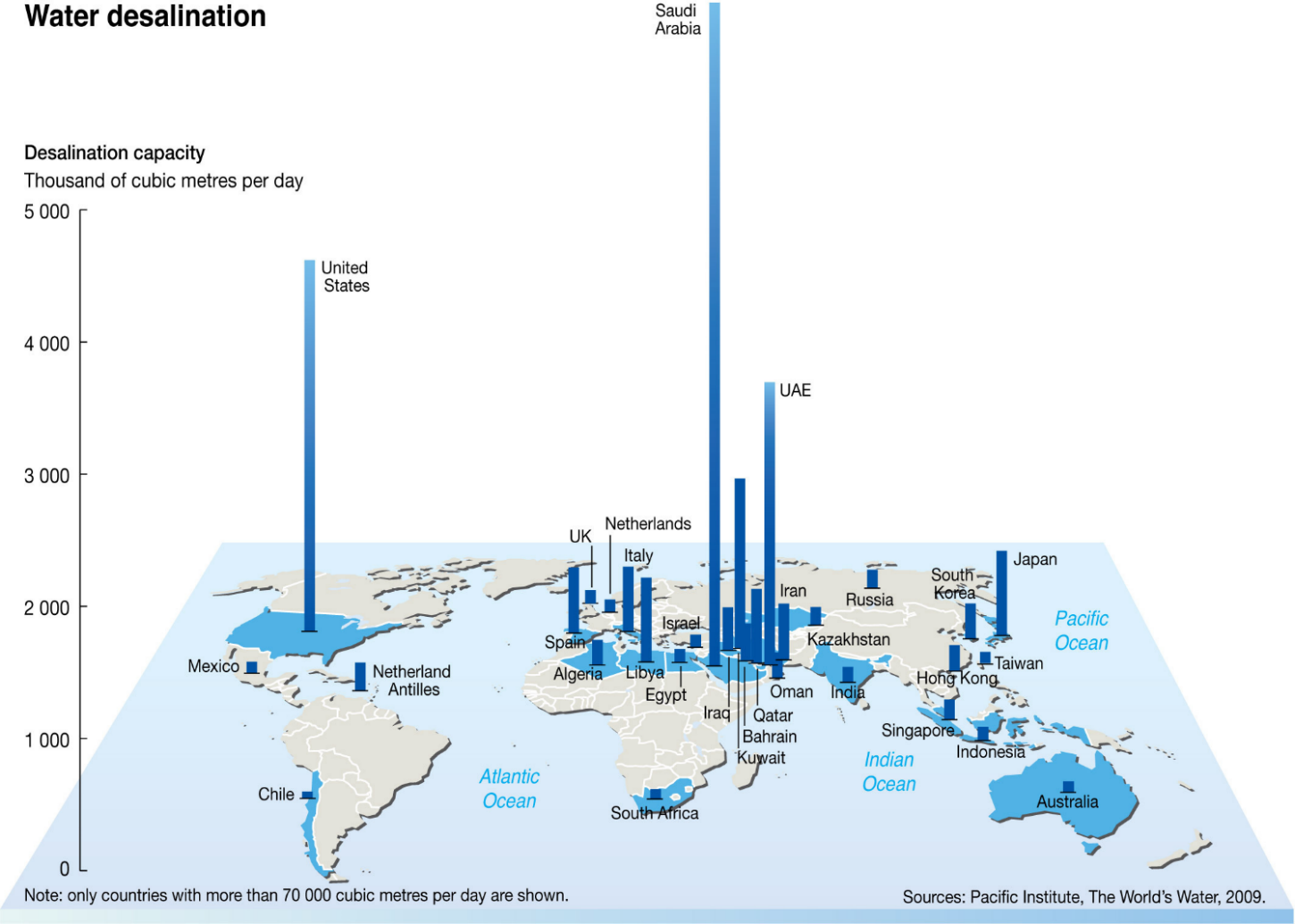
Seawater desalination plants in the word
If you see the global seawater desalination capacity, you can see that they’re located in the US, Middle East, in particular, Saudi Arabia is the largest desalination plant, Australia and we have several major desalination plants.
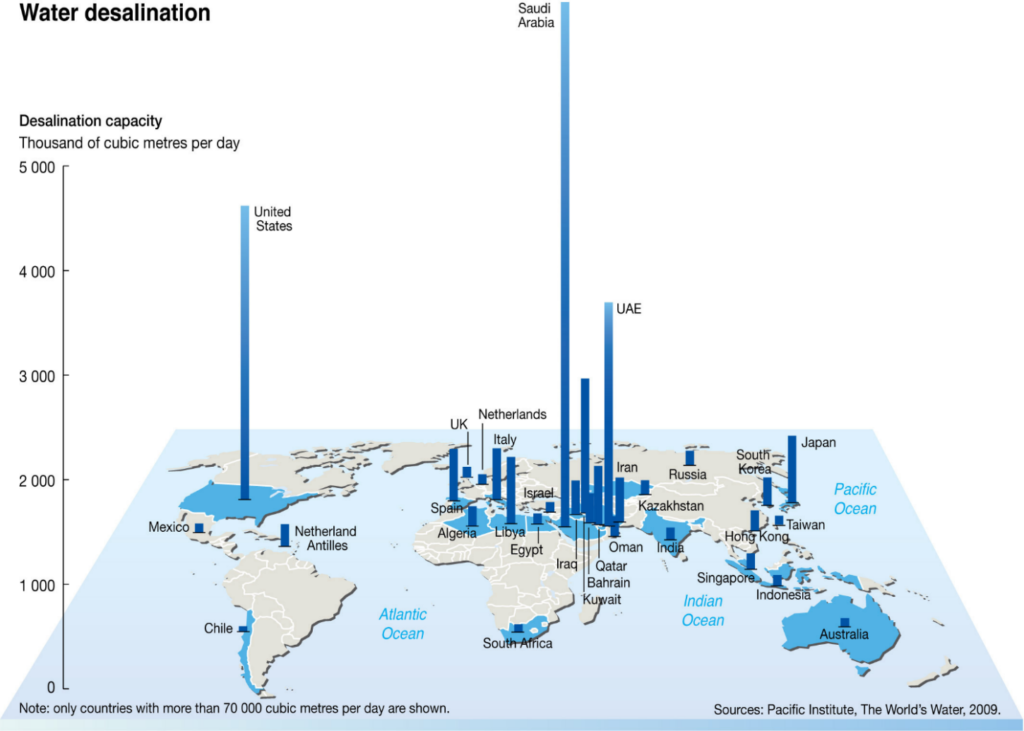
So kept the cost of the desalination plant since 2013, it keeps increasing because most people want to live in near the sea and then they have urbanisation in the other way around the seawater and we need more water like Sydney, Melbourne, Brisbane, Perth, and Adelaide. We are all located in near ocean, which will be a very important water resource to responding to the growing population.
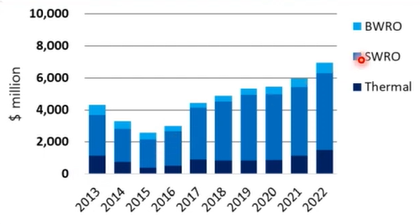
So, we need to learn what’s the seawater desalination.
Seawater desalination in Australia
This is the example of desalination in Australia. So, this is the timeline of the desalination. you can see all the detail and 2008.
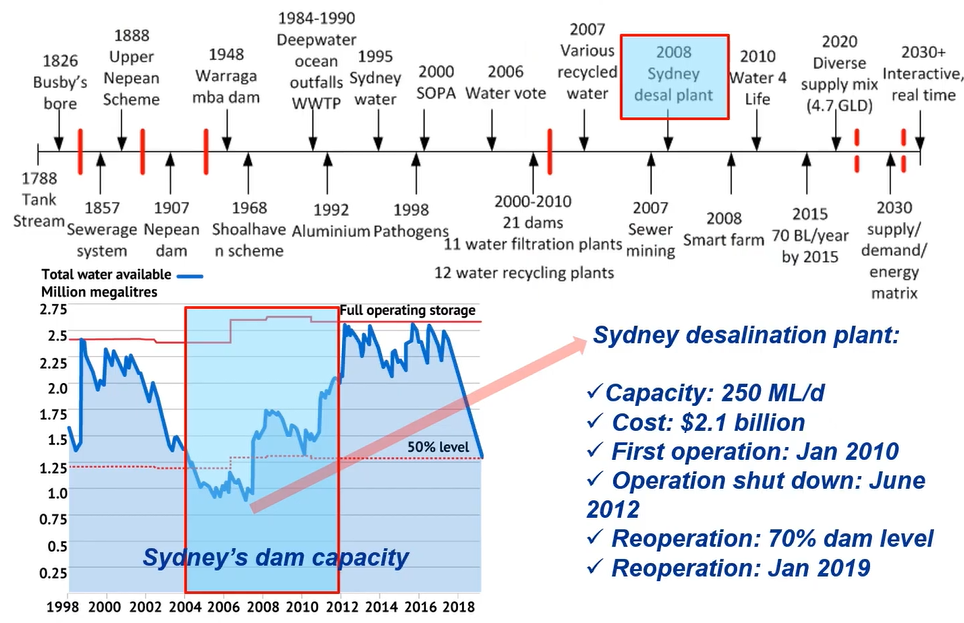
Sydney desalination plan was initiated because of Sydney dam capacity. You can see 2008 in above diagram. Dam, level was lower than 50% and then we need to look at the alternative water resource that’s the reason why we constructed a Sydney desalination plant, and this capacity is 250 Mega litre per day, and it costed $2.1 billion.
We first operated it in January 2010 and operations was shut down in June 2012, because we had the enough rainfall and then never increased almost 99% that’s the reason why it was completely shut down. Because this desalination plant requires a lot of energy and were operated when we had 70% of dam level and it was January 2019. So, depending on the dam level, we have the operation and shutdown for our desalination.
Big desalinations plant in Australia
We have 6 desalination plant gold coast, Sydney, Victoria, Adelaide, Perth, and Southern seawater desalination plant.
These are the major seawater desalination plant in Australia. In the future as population grow even Newcastle, Wollongong and then another major city may require big seawater desalination plants to sustain their water resources, the mineralization.
Sydney seawater desalination plant
In Sydney desalination plant have the capacity is 250 Mega litre per day.
This process consists of
- Drum screens
- Dual media gravity
- Micron cartridge filtration
Here micro cartridge filtration, just try to protect.
- The 2- pass seawater reverse osmosis (SWRO)
Just in case large particle pass through the descriptive filtration and then try to reduce the membrane fouling to reverse osmosis system and
- Post treatment is lime, fluoride, and CO2 remineralization to compare to the other plant.
So, post treatment, there is the one more special post-treatment for all right. We can also think about even though we have 6 desalination plant, they are a bit different. However, the most common processes the seawater reverse osmosis, RO process is very consistent.
We have to learn how to design this kind of desalination plant, this is not only seawater desalination plant. Reverse osmosis (RO) can be used for the wastewater, also industrial water, use mining water reuse. So, reverse osmosis is a very well-established technology for recycling water because this can remove all the salt and contaminate. For this desalination plant briefly, and let’s look at the big more detail how this desalination plant has to be designed and it will work.
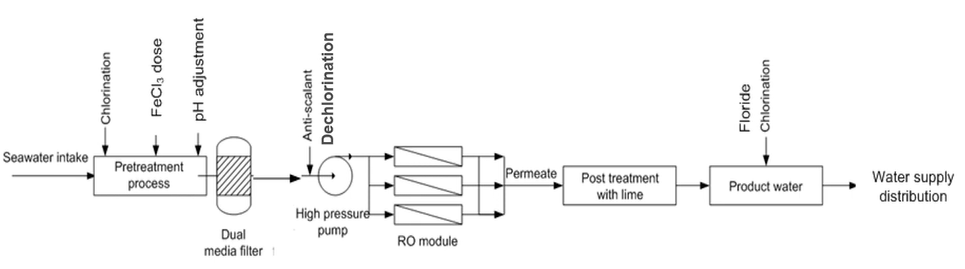
You can see this is the desalination plant general schematic diagram. This is the exact same process schematic of Sydney and quality cost desalination plant. So, two desalination plant was designed by the Veolia Water and then they made a very similar process.
let’s look at one by one and then we can better understand how to work and design better for this process. So, already you have experienced the taste of seawater its very salty, because the salt concentration is almost 3.5%, or 35 gramme per litre. So where is this salt from? When this seawater concentration is very high. When the earth was born 100 million ago at the time we had on a volcano, and then everything was just a born and then we had a lot of raining.
So, this raining, just to watch the hour or all the dissolved of the salt, such as sodium chloride, Magnesium, Potassium, and any kind of dissolved the salt and organics. That’s the reason why seawater was 35 gramme per litre, when the earth was born and then still, let’s see water the concentration is very much similar. So, this seawater salt concentration is problematic, because when you design this high salt, we have to remove this small molecule. So that we have to spend a lot of energy.
We need to go through first this screening system for the pre filtration and chlorination.
Chlorination in the desalination plant
The chlorine kills bacteria. So, microorganism will die because the chlorination so in the process, pumping, piping, so we want to make sure that all the biofilms will never ever grow.
So, performance we’ll be maintaining very sustainably, and chlorine will be added to remove all the microorganism and biofilm inhibition.
Ferric chloride (FeCl3) dose
After chlorination we will add ferric chloride (FeCl3). So ferric chloride will be dosed, because large particle can be influent this process which will clog the media filter reverse osmosis.
So, we need to remove the large particle so flocculation using Iron chloride, so, we can try to remove and settling down however, mostly the sea water quality of large particle is very clear, the heavily contaminated by the large particles. When you have a very large concentration of turbidity you can apply for this Iron chloride concentration.
PH Adjustment
So, what’s the pH of water so, let’s just see our drinking water. Raw water the pH around 6.5 to 7.5 near a neutral pH and what’s the pH of Coca Cola.? Coca Cola and lemonade pH is around 2. So, pH is very low, and it allows cavity causing bacteria to grow and develop that’s the reason why when you have your teeth for long time is Coca Cola and lemonade.
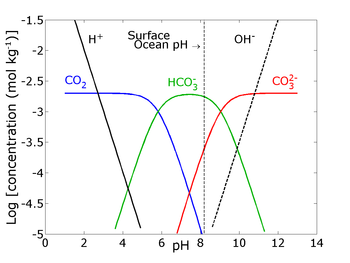
We must consider what’s the pH of the seawater. So, this pH has seawater is around 8 or 9.
In the seawater there are dissolved the inorganic compounds, which is the total of aqueous CO2 and bicarbonate (HCO3–) and carbonate (CO3-2). So, this CO2 concentration in the air they are key exchange their concentration with seawater so that they have a different pH variation depending on what kind of the you have a dominant species become a among this CO2, bicarbonate, and carbonate.
You can see the CO2 concentration. We have a model pH 8. So, you don’t have any more CO2 concentration. However, when you have PH 8, so this bicarbonate has the highest concentration, when you have a pH more than 9 So, this carbonate concentration is the highest.
So, this is the reason why the seawater has very high concentration of bicarbonate and then this creates the good buffer solution and create a very high pH of the seawater, the carbonate ion concentration increases with increasing pH and when more CO2 dissolves in seawater, it becomes more acidic.
Let’s think about climate change. We increase the CO2 concentration, which means CO2 will dissolve more in the seawater. So, seawater CO2 concentration increase it becomes more acidic condition which you will be changing the seawater aqua system for example, like a shares, Craps, or Kores. So, they need the creation to create the skills or their lives and when you have a high pH and they can produce the CO2, bicarbonate, carbonate and then produce the calcium carbonate with their skins and corals.
However, when you have a very high concentration of CO2 due to the climate change, and the seawater becomes more acidic which mean the coral cannot grow and shares, crops cannot survive in the seawater that’s the reason why the pH of seawater depending on also CO2, bicarbonate, carbonate consideration. This pH is relatively high, which will create the inorganic scaling and fouling that’s the reason why we need to make sure lower the pH.
So, at pH 8, we must put hydrogen sulphate to reduce the pH up to 8 which will make better performance of this dual media filter, reverse osmosis process. So, pH has to be reduced to 6 based on the original pH of 8.
Dual Media Filtration
Next step is to dual media filtration and pre-treatment. So, we need to remove some of the particle in the seawater. So, this dual media filtration is like a sand filter or some ultra-filtration system. So, you can have a see water the pass through this media filter which will remove most of large particle and called for the reverse osmosis process.
Conventional and ultra-filtration pre-treatment
So, there are two different pre-treatment process one is conventional, which is dual media filter. Sydney desalination plant, Victoria desalination plant and Perth desalinization plan have conventional filtration So, far of the desalination plant consists of this dual media filtration we call conventional pre-treatment and southern desalination plant, Adelaide desalination plant include ultra-filtration pre-treatment. So, two plan consists of ultra-filtration advanced pre-treatment and footprint Conventional pre-treatment.
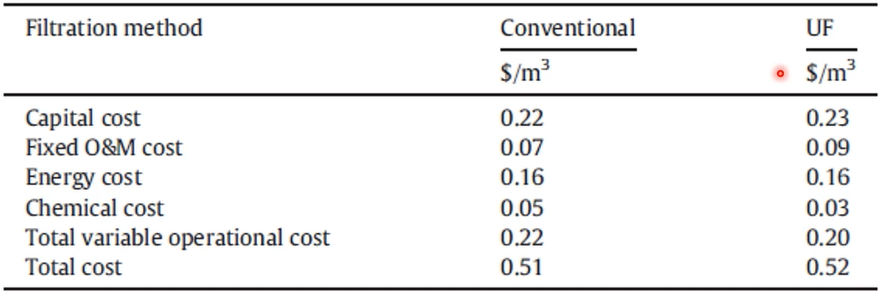
So, when you’re considering they’re all the capital costs, when an operation and maintenance cost, energy costs, capital cost, so, product cost is pretty much similar, but people believe with ultra filtration are the best retreatment is better quality of life for pre-treat water, and then very consistent performance of reverse osmosis that’s why people like to have more ultra filtration pre-treatment.
But conventional little bit cost effective or it’s easy to maintain, easy to handle and operate that’s the advantages of this pre-treatment system.
Anti-scalant addition
After pre-treatment, your media filter and just the further reverse osmosis. You need to go through anti-scalant. So, we have a seawater, and we need to extract water using the RO which mean your concentration of Calcium, Magnesium, silica, which are the very dominant inorganic scaling compounds, so, you can see the calcium carbonate because of the pure water the extracted your concentration of cation and CaCO3 increased, and you will have crystal on the surface of the outer membrane was Calcium sulphate and magnesium carbonate, even cation phosphate.
There are many different inorganics scaling and because of low solubility, and they try to create the crystals. So, these crystals will be a problem to crop the reverse osmosis membrane. So, we need to add anti scalant, which sulphates active materials that interfere with precipitation reactions in three primary ways.
- Threshold inhibition,
- Crystal modification
- Dispersion
So, anti scalant tries to interfere your precipitation reaction from these 3 ways. In seawater desalination, we have a commonly used the anti scalant, which is sodium hexametaphosphate (NaPO3)6.
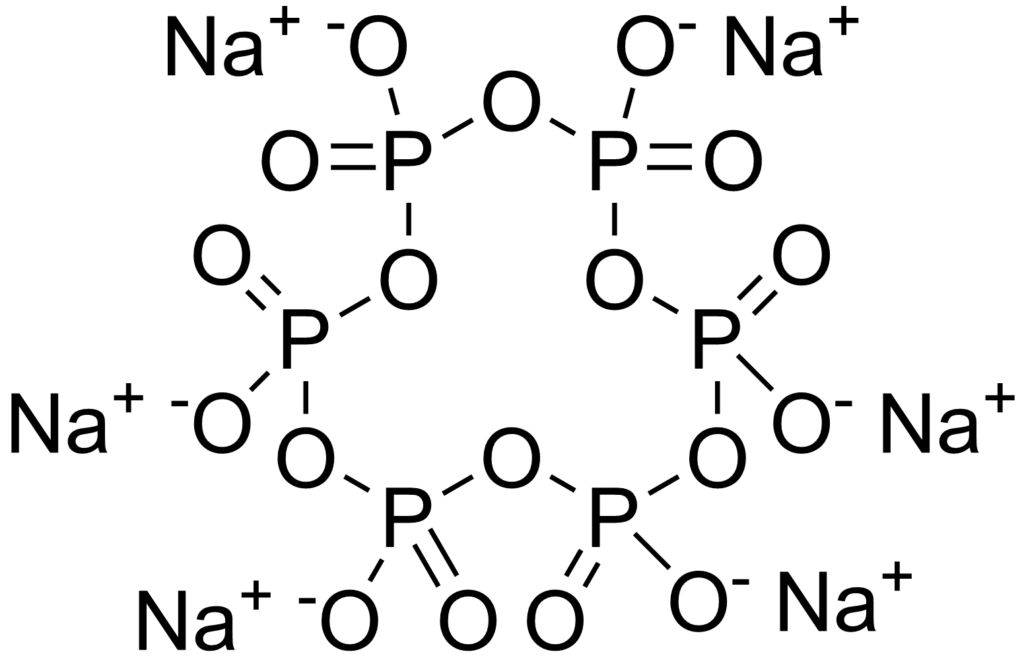
So, this composition can capture your calcium, magnesium and some of inorganic scaling and primary compound and try to reduce your inorganic scaling. So, above diagram is the structure of this sodium hexametaphosphate. After you add this one, we need to go through the de-chlorination.
So, what’s the dichlorination? So, we have the chlorination here to remove microorganisms and bacteria to inhibit biofilm in the palm pipeline and filtration system, and we have a de-chlorination.
De-chlorination
The de-chlorination process just to remove chlorine. So, why do we have to remove chlorine because the reverse osmosis membrane is polymer, which is the polyamide. So, this polymer will be degradable when you have oxygen. So, chlorine is a very strong oxidant which will degrade your polymer surface.
De-chlorination can be processed by 2 methods
- Addition of sodium (meta) bisulfite (SBS)
- Granulated activated carbon filter
So, normally in seawater desalination we don’t have to go through another granulator carbon filter, because this chemisorption process where chlorine there with the carbon sulphate, which will make active chlorine to chloride ions. So, this chloride ion is no longer oxidants So, this is the very safe for reverse osmosis. However, this cannot be used for this dechlorinated in seawater desalination, we just put the SBS.
So, dosing ratio is around 3 to 1 ratio of SBS:Cl2 to active chlorine dose. So, reaction time is about two minutes. So, this is the SBS and chlorine together with water and sodium sulphate and then produce the hypochlorite.
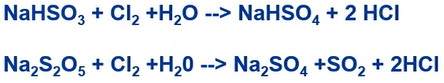
In granulated activated carbon filter, the compounds Cl2 is strong oxidant but becomes just a normal sort of chloride iron was this is the similar reaction. So, we have chlorine active material to iron salt. So, that’s the reason why we need de-chlorination process.
Reverse Osmosis (RO) in desalination plant
The reverse osmosis, which is the most important because in seawater desalination, wastewater reuse, and industrial wastewater process all consists of reverse osmosis, because they remove basically everything after this reverse osmosis membrane.
After reverse osmosis most of salt removed and the pure water is collected and desalinated water go to post-treatment with lime so, we just call de-mineralization. So, why do we have to go through de-mineralization because we remove the all the minerals from seawater after reverse osmosis. So, low mineral water has pure adverse effects.
first one is the very high corrosion potential. So, if you have no minerals such as calcium magnesium, so, when you carry your water desalinated water in the pipeline. This has no mineral and no buffer and very corrosive potential.
second reason is that dietary deficiency causing risk of ischaemic heart disease and some vascular disease.
Two solutions are widely used to remineralise /neutralise water
- CO2 addition + Calcite limestone (CaCO3, MgO) Percolation + Na2CO3
- Addition of CaCl2 + NaHCO3
We put the calcite limestone. calcite limestone, it’s like stone and then filtration and just to dissolve the calcium and we can have some good remineralized water and CO2 can also create the good proper solution. The CO2 depending on pH become bicarbonate, carbonate, which will create a higher alkalinity and proper capacity of water, and this gives the good performance of RO less corrosive potential. Addition of calcium chloride and sodium bicarbonate can be remineralized compounds.
Fluoride and chlorination
Fluoride is used to mainly in Sydney desalination plant. Fluoride can reduce the cavity in your teeth. So, this fluoride is an additional compound, which can protect our teeth and then we have another chlorination to remove some microorganism while RO remineralise water carries to the pipeline.
Operation and Management Cost
The 1st one is energy. So, 51% of energy cost and then you have indirect cost like monitoring maintenance, labour and waste stream disposal, chemicals, and membrane replacement etc.
So, energy cost is very high in desalination treatment as compared to wastewater re-use treatment, because of high salt concentration, which mean high osmotic pressure to overcome this high osmotic pressure, we need a high-pressure pump, which is equivalent to energy consumption.
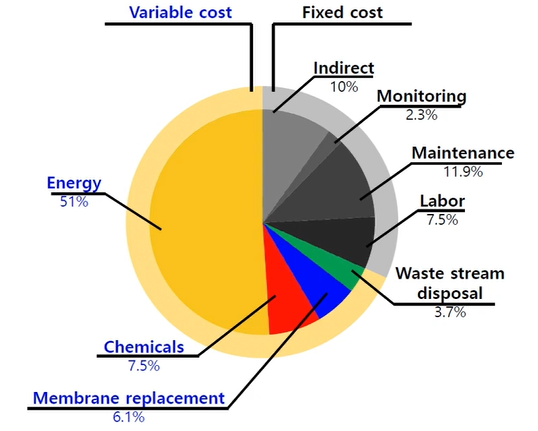
We need around 60 bar, high pressure for our reverse osmosis membrane, which will recover around 50% of desalination water. So, that’s the reason why energy cost is very, very high. So, when you produce the one cubic metre of seawater, desalinated water, energy requirements are the 3.5 to 4-kwh.
So, how much it cost for one kilowatt hour in Sydney. So, it costs around 0.3$ for one kilowatt hour energy costs, which mean for kilowatt hour for one cubic metre. So, you need around 1.2$ for one cubic metre of desalinated water for only energy cost.
So, this energy cost is very high and then we need to save how to reduce this energy for desalination plant. So that’s the overall our future area, which can contribute very significant desalination plant.
Quality Parameters for membrane process
Essential water quality parameters are very important to design or run a seawater desalination plant because, we can make a better selection of pre-treatment and RO membrane was we can predict organic fouling, inorganic fouling, and biofouling. So, let’s look at the sea water quality parameters.
Turbidity
Turbidity levels above 0.1 milligrams per litre are indicative of high potential for fouling and spikes above 50 NTU for more than one hour would require sedimentation, pre-treatment, or dissolved air flotation treatment (DAF) prior to filtration. So, high NTU will damage the pre-treatment so, that we must utilise the DAF or flocculation sedimentation process before filtration process.
Total Organic Compounds
So, if you think about the summertime and wintertime, so, our microbial activities more active during summertime and we have more organic compounds. So, if below 0.5 milligrams per letter biofouling is unlikely. So, above 2 milligrams per letter biofouling is likely. During the summertime more organic concentration So, biofouling effect will be much higher than wintertime. Its depending on the area, we must consider this total organic carbon concentration in general in Sydney we have almost less than 1 milligrams per litre of total organic carbon concentration, which is relatively safe for biofouling.
Silt Density Index (SDI15)
The level is consistently below two years around typically indicate that no additional filtration pre-treatment needed and SDI greater than 4 means pre-treatment is necessary. So, you can measure SDI by following formula
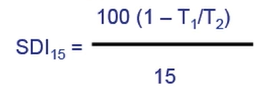
T1 is the time in second to filter initial 500 millimetre of sample. So, sea water initially 500 millimetres collected, and then how many seconds we measure.
T2 is time in second to filter final 500 millimetre of sample. After 15 minutes allowed to pass between timed sample intervals. First 500 millimetre and after 15 minutes and we measure again T2 in seconds to filter final 500-millimetre sample and utilise this equation.
When you have more than 4 of the SDI, you have very bad water quality. You need pre-treatment to reduce the SDI and protect the reverse osmosis. So, this SDI is important when you have RO membrane warranty. So, if you have a high SDI, very bad water quality, which will lead to causing your RO membrane and pre-treatment.
So, when you have a contract with membrane company, they try to make sure warranty when you have last year SDI more than 5, they don’t have any warranty period. However, you have water quality is less than 5 for four SDI. So, they can have a good warranty timing when you have your feet of water quality
Total suspended Solid
Needed to access the amount of residue is generated during pre-treatment. So, does not correlate well with turbidity and beyond 5 NTU
Iron concentration
Iron concentration is one of the essential parameters because if Iron is in reduced form, to sea water the RO membrane can tolerate up to 2 milligrams per litre, if Iron is in oxidised form like iron oxide, every Fe+2 or Fe+3 and concentration more than 0.05 milligrams per litre would cause accelerating fouling.
Manganese Silica Concentration
Manganese is very much similar if manganese is reduced from, SWRO a membrane can tolerate up to 0.1. If manganese is oxidised from concentration more than 0.02 milligrams per litre hold cause accelerate the fouling. So, when you remove this kind of Magnetic Iron, we need the extra treatment such as coagulation process.
Silica concentration higher than 20 milligrams per litre may cause accelerated fouling. So, you need to analyse colloidal silica concentration. If concentration is more than 20 milligrams per litre. So, we must consider what kind of pre-treatment process is required for silica removal.
Chlorine Concentration and Temperature
Chlorine concentration higher than 0.01 milligrams per litre cause RO membrane damage as chlorine is oxidisation So, can damage the RO membrane polymer.
- Temperature less than 12 degree would cause a sudden increase in unit energy use
- Temperature more than 35 degree may cause accelerated mineral scaling and biofouling
- Temperature greater 45 degree may cause RO membrane damage
Additional parameters
An additional parameter also needs to consider like carbon dioxide, bicarbonate, and carbonate. So, this is the buffering capacity which will combine with the calcium carbonate post-paid and the manganese carbonate which will generate in organic scaling extract the alkalinity, hardness such as magnesium and calcium Phosphorous.
Total dissolved solids which are related to osmotic pressure. So, sea water has 35 gramme per litre. We must consider how much hydraulic pressure must be applied, and that recovery can be obtained, and the same factors are very important for designing your seawater desalination plants.
SWRO History
First seawater desalination in Kuwait 1956 and desalination, commercial plant construct construction 1970 and we had the leading desalination market at the time by distillation.
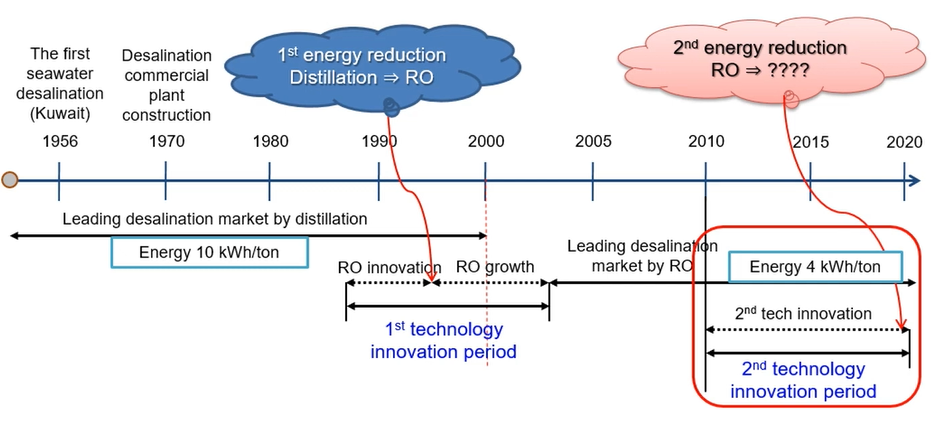
So, problem was the energy consumption was very high 10-kilowatt hour per cubic metre. So, just boiling and then your energy consumption is almost more than double of reverse osmosis (RO)
Firstly, energy reduction desalination to reverse osmosis happen only 1992 – 2000 so, reverse osmosis membrane innovation and then growth. So, this is the first technology innovation period and since 2005 So, a lot of commercial opportunity and produce the leading desalination market by reverse osmosis and as energy costs almost 4-kilowatt hour per cubic metre.
So, this is the second technology innovation. So, look at the distillation 10 and nowadays we can operate energy reduction by up to 4-kilowatt hour/m3. So, we can study for reverse osmosis, how to design and operate better rather than this.
NF/RO membrane
These are the general size and different contaminants. Human hairs 10 to 100 micrometre and 1 millimetre to 100 micrometres activate carbon.
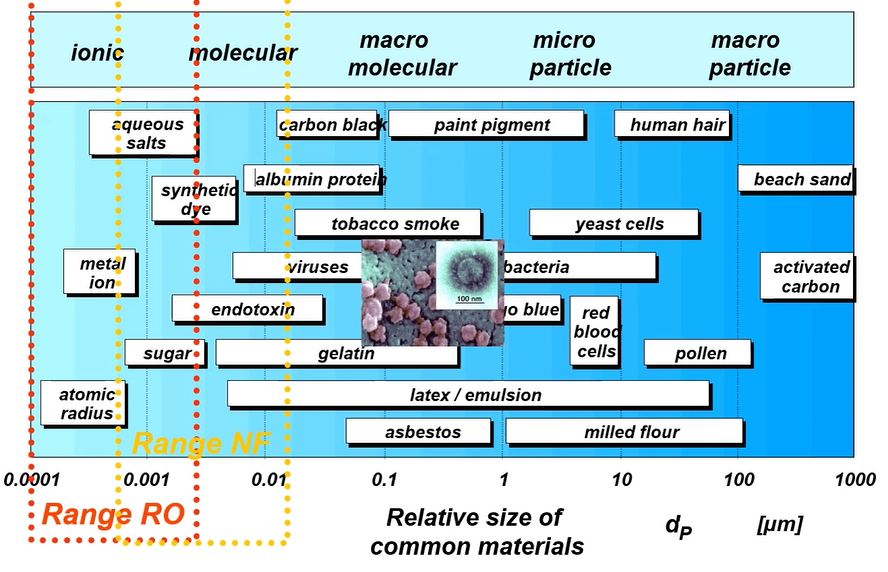
So, this is the range of nano filtration and reverse osmosis. Now there’s Coronavirus is one of the big issues. So, virus sizes are 0.005 micrometre to 0.1 micrometre. So, Coronavirus is around 100 nanometres size 0.1 micrometre. So, when you remove this virus, so, we need to utilise the ultra-filtration or nano filtration.
So, above diagram clearly mentioned the nanofiltration range. Nano filtration can remove sugar and then some of the other toxin compound, dies. and when you try to remove the sodium chloride, which is the most abundant concentration in seawater, salt, the metal ions and atomic radius.
Sodium size is almost 0.6 nanometres and chlorides a bit bigger size then if we separate from pure water H2O less than 0.6 nanometres size and then we must utilise the reverse osmosis membrane. We must design this kind of membrane to separate your sodium chloride from seawater and only pure water can be extracted.
RO membrane nano filtration very much similar mechanism small nonporous membrane. membrane thickness increased permeate flux decrease the active layer as soon as possible to produce the higher flux and membrane structures are symmetric.
TFC NF/RO Membrane
The RO membrane element and membrane structure. So, you can see this is RO element. So, you have a lot of layers. So, we just call spiral round membrane element, and the microscope membrane structure. So, you can see the there are three layers for RO membrane.
- First layer is ultra dense skin layer,
- Second layer is Polysulfon-support layer
- Last layer is the Backing
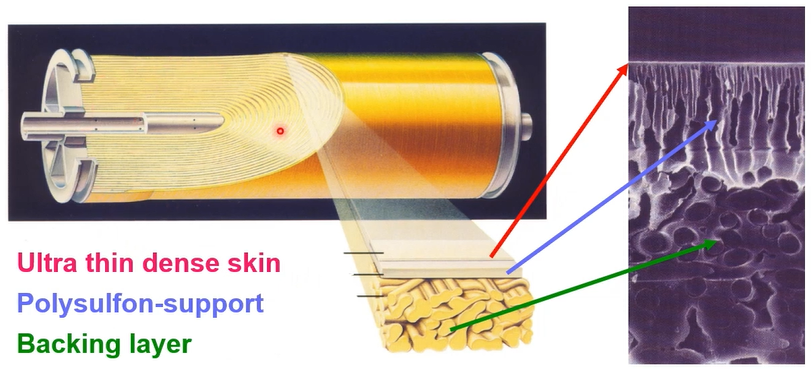
So, you can see that this thin layer we just call polyamide composite. we just call the TFC thin composite RO membrane. This skin layer polyamide can separate your salt and pure water.
The pore size is almost 0.6 nanometre and water molecule will pass through and then you can separate your salt and pure water. So, this skin layer is very important.
However, this skin layer is 20 nanometres of thickness. So better way that we need to have support layer for the Polysulfon polymer. So just Polysulfon and then we pressurise the 60 bars. So, this pressure must be tolerated from this membrane. That’s the reason why we have certain layer of the backing layer.
If we look at more detail. The 20 nanometre of skin layer, active layer and other the Polysulfon support layer and next is the nano backing layer. So, 3 layers consists of RO membrane.
Transport Phenomena
Next is the transport phenomena of reverse osmosis membrane
- Mass transport across membranes that diffusion
- Osmosis is very important. So, we need to understand better of osmosis
- Concentration, polarisation
- Permanent flux
Which is emitted by osmotic pressure and concentration propagation apart from the intrinsic the membrane resistance.
Osmotic Pressure
We have a feedwater very low concentration of salt and brine, very high concentration of salt in between we have RO membrane. So, when you have this condition, what happened is that then what the pure water low salt concentration will pass through your membrane, and then you have water head. So, your high concentration, high osmotic pressure will suck your water molecule through your RO membrane, and then you can create your natural osmotic pressure. So, we just call for those osmosis.
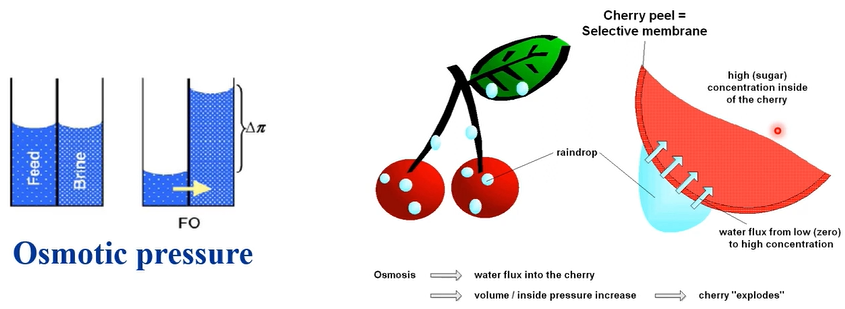
Opposite meaning is reverse osmosis. So, we’ve pressurised here, and then pushes through your water molecule from the lower concentration to the higher and we just call reverse osmosis. So, when you look at the nature, so what kind of osmotic pressure can be applied? So, this is cherry. So, cherry has very high sugar inside your fruits and then sugar has high concentration and high osmotic pressure.
So, when you have water, rainwater and what will stay on this phase of cherry and this water molecule then skin layer of the cherry is almost membrane. So, water molecule will pass through because of the sugar osmotic pressure and then you can burst out this cherry if you have very high concentration of your sugar because what molecule with a pathway and then make them bigger and bigger finally, this cherry will be burst.
Let’s look at the compound’s sodium chloride, magnesium sulphate, sucrose, and sea water. When you have a sodium chloride 1000 milligrams per litre and osmotic pressure is 100 kilo Pascal which is 1 bar and so, magnesium sulphate was 1000 which is a 25 kilo Pascal 0.25 bar and sucrose is sugar which is similar to cherry sugar concentration has very low osmotic pressure because this is the organics salt.
So, it’s not fully ionised in water. So, that was not pressure is relatively low and then sea water 35000 milligramme per litre has around 27 bar or osmotic pressure.
![]()
So, when you have a high concentration, you have a very high osmotic pressure and idea constant is fixed and temperature is the solution. So, when you have high temperature, you have a high osmotic pressure because you can dissolve your soul and solubility is higher, we have to be considered what’s the dissociated solid.
This is the osmotic pressure where from your recovery way. So, we have a feedwater 36 gramme per litre of salt concentration and this is the RO membrane for already 50% of the recovery the flow rate of feedwater and flow rate brine is caused to this way and then what the molecule passed through, and we have almost less than 250 milligrams per litre of permeate concentration.
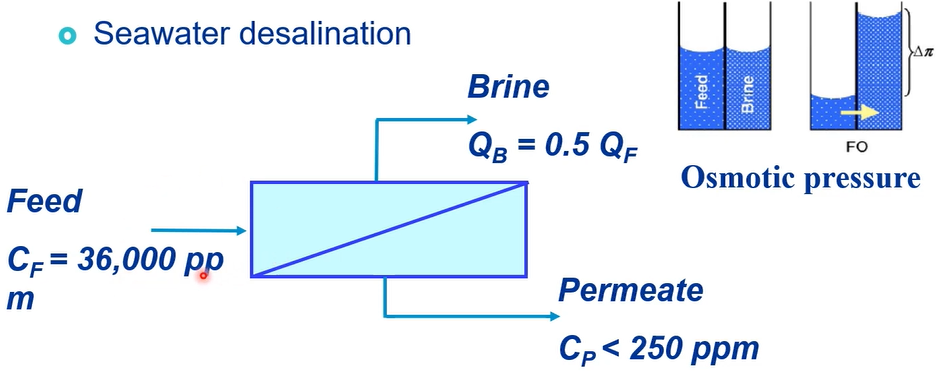
So, this is the osmotic pressures 30 bar and then your osmotic the pressure is almost 60 bar in brine because this is the 50% of the recovery. So, energy consumption three times the theoretical limit 3.5-kilowatt hour per cubic metre. So, reverse osmosis recovery can be calculated based on permeate flow over feed flow
Concentration Polarities (CP)
So, this is the unique terminology for reverse osmosis membrane. look solute concentrate at the membrane surface is higher than bulk concentration or distance away from the membrane surface. Diffusion from the membrane surface back to the bulk solution. So, back diffusion can be enhanced by crossflow high shear rate. So, let’s say this is sea water and salt concentration.
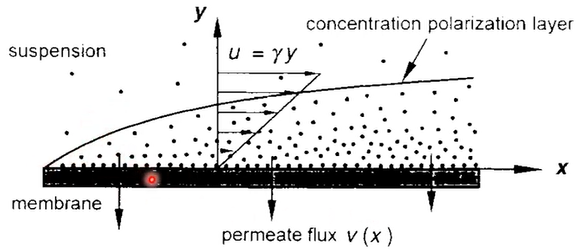
So, pure water will pass through this membrane, and you have accumulated a salt and then higher concentration of your salt. So, with your membrane less your concentration polarisation layer of your salt concentration is higher. So, the bulk concentration and then you have a membrane surface concentration. So, depending on the concentration polarization layer, your bar osmotic pressure, real concentration of your osmotic pressure is different from bulk osmotic pressure. So, we need to consider this concentration polarisation to better design your osmotic pressure between your membrane feed side and permeate side.
So, a real osmotic pressure difference will occur between this membrane and then concentration polymerization will be a real or somatic pressure your design of seawater desalination so, all RO process is in cross promote today make this CP effect. So, you have a very high cross flow then you will wash out and make a turbulent and reduce the high concentration of your salt on the membrane surface.
NF/RO Membrane
So, let’s look at the more detail how we design this RO element.
Pressure vessels
- Pressure vessels consists of fibreglass or stainless steel to tolerate high pressure more than 60 Bar, or 70 Bar. So, we need a very strong material
- Up to 8 spiral wound elements in a vessels
So, if you look at below diagram, outside is the pressure vessels. So, inside pressure vessels each element will be located. So, your feed water is passed through and in between. So, you’ll sea water flowing this channel flow and then you have permeated, water flux can be collected from this product what the outline then this is the cube concentrate in then brine is coming up from this pressure vessels and this required very high pressure.
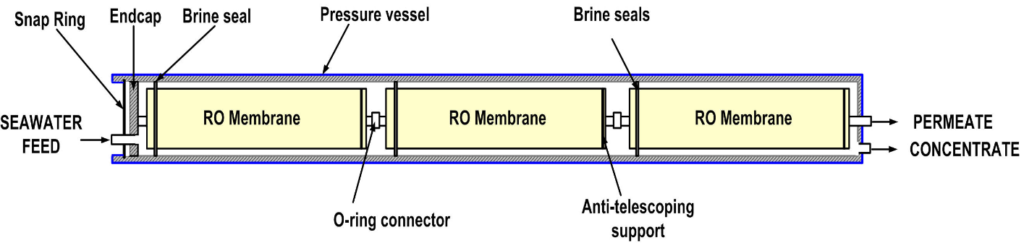
So, you can design very high strong material, and this can be a good kind of design and we can put around 1 to 8 elements in one pressure vessel. So, depending on the design capacity. We can also select 7 elements or 6 elements.
Energy Distribution
let’s look at the energy distribution in seawater desalination plant. So, this is the overall total energy uses 3.9-kilowatt hour/m3 required to desalinate water with TDs concentrations 35 gramme per litre and temperature 23 degree. Intake from seawater, we collect sea water, so we need a pump. This will consume around 0.2-kilowatt hour per cubic metre, and this goes to the pre-treatment filtration or sand filtration or ultra filtration.
So, we need to pump and pressure 0.4-kilowatt hour/m3 for pre-treatment. So, almost 10% of the energy consumption in the desalination plant have to pre-treatment near the high-pressure pump to operate the reverse osmosis system.
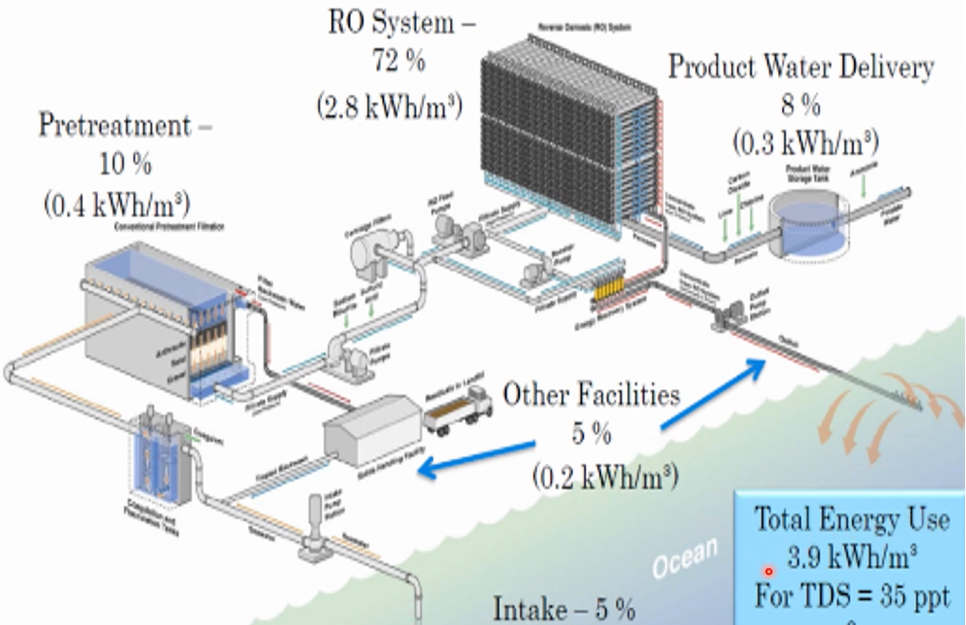
This RO requires 2.8 kilowatt hours/m3 of energy consumption, which is equivalent to 72% of energy consumption and After pumping, we must deliver this water. Delivery pumping energy consumption is 0.3-kilowatt hour per cubic metre, almost 8% of overall energy consumption. We have added facilities such as the sludge disposal and then discharge to our brine and we need around 0.2-kilowatt hour/m3 almost 5% So, all together 3.9-kilowatt hour/m3.
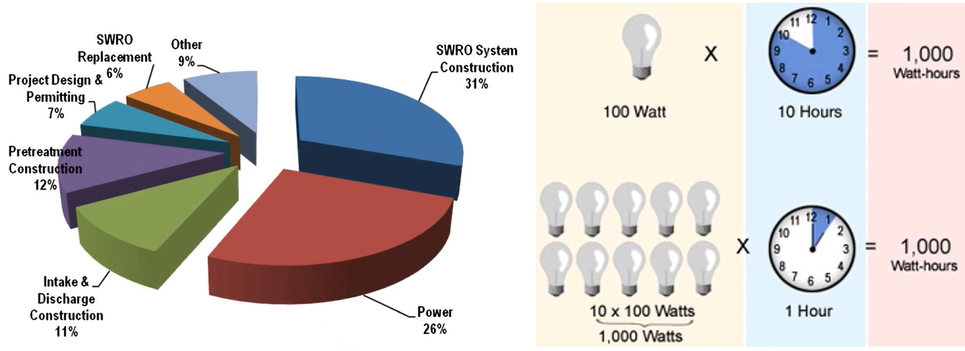
Let’s look at the overall energy So, this is the seaward RO system construction cost 31%, Power cost 26%, intake discharge construction 11% , pre-treatment concentration 2%, SWRO membrane replacement 6%, others chemical or sludge disposal 9%. Let’s say these 100 kilowatts, what times 10 hour we just say 1000-watt hour. So, this is 10 light bulbs and 100 Watt utilise 1000 watts per one hour is equivalent to 1000-Watt hour.
So, one kilowatt hour energy cost is almost 0.29$ in Australia. So, let’s say 4-kilowatt hour per cubic metre disseminate. The water energy cost of Sydney desalination plant, we produce the 250,000 m3/day. So, every day, we spend $290,000 per day for the energy consumption, which is very huge destiny, why all the desalination plants in Australia utilise the wind farm, which is the renewable energy to reduce the CO2 and the environmental impact.
Sea Water Desalination Frequently Asked Questions
1) Which Australian city uses the most desalinated water?
Up until now, desalination has been mostly used in Perth and Adelaide. What is the biggest desalination plant in Australia?
The largest desalination facility in Australia is located at Wonthaggi. An underground conduit transports the Wonthaggi treated water to Melbourne’s water supply system. aerial shot of the building site for the plant. The plant operates entirely on renewable energy.
2) How does chlorination water treatment work?
How is chlorine used for disinfection? By severing the chemical connections in pathogens’ molecules, such as bacteria and viruses, chlorine can destroy them. Chlorine compounds, which may exchange atoms with other compounds like the enzymes in bacteria and other organisms, make up the disinfectants employed for this purpose.
3) Is reverse osmosis and desalination the same?
Desalination, generally known as the process of removing salt from seawater, is a function of reverse osmosis. To remove salt and other pollutants from water, it employs high pressure and a semipermeable membrane.
4) Does temperature affect chlorine concentration?
It makes sense that chlorine depletes more quickly in warmer water because all chemical reactions can proceed more quickly in warmer environments.
5) At what temperature does chlorine become ineffective?
Even at temperatures as low as 65°F (18°C) and as high as 99°F (37°C), chlorine is quite effective. When the water temperature falls below 75°F (24°C), bromine is less effective.