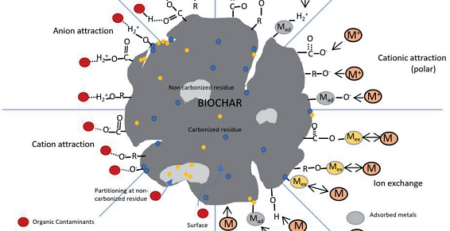
Use of Nanoparticles in Water Purification
Nanotechnology for Water Treatment Application
A conventional water treatment becomes a bit difficult to remove all the pollutants, because of the factor urbanisation and industrialization.
So, nanotechnology has been used to resolve these problems in water treatment applications, which is this technology associated with the reduction of pollutants such as bacteria, hazardous heavy metals, pesticides, and other persistent and toxic chemicals.
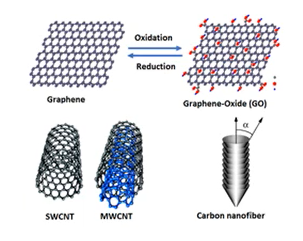

In addition, nanomaterials are developed with technologies that are very helpful in adsorption, sensory, and catalysis such as reactivity, electrostatic, high surface area, hydrophobic and hydrophilic. The overview of the nanotechnology that has been used in water treatment applications.
As Nano adsorbents for wastewater treatment manufacturer, Nanotechnology is known as highly effective, flexible, and multipurpose in nature. It also provides a greater performance, which is an efficient solution for water treatment. Nanotechnology better alternative for removing the contaminants such as heavy metals, oily water suppression, and anti-microbial activity in order to improve the quality of the water.
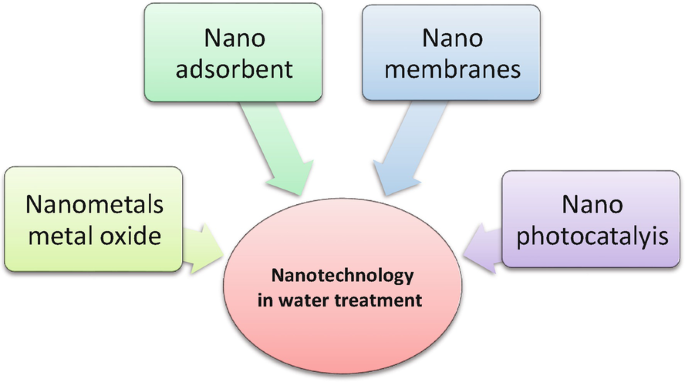
According to the nanomaterials, nanotechnology of water treatment is divided into four main groups which are
- Nano Photocatalysis
- Nano Adsorbents
- Nano Membranes.
- Nano metals or Metal oxides
 In addition, in industrial water treatment, the nanomaterials that usually been used are carbon nanotubes
In addition, in industrial water treatment, the nanomaterials that usually been used are carbon nanotubes
- Graphene oxide
- Nanoparticles, fluorinate silica nanoparticles
- Layer nanomaterials.
Type of Nanomaterials
The type of nanomaterials that have been used in water treatment are
- Dendrimers
- Zeolite,
- Nano sorbents
- Nano catalyst
- Metal nanoparticles and
- Carbonaceous nanomaterials
 Benefits of Nanomaterials
Benefits of Nanomaterials
The benefit of using nanomaterial only less pressure is needed to pass the water through the filter by using nanofiltration methods. It is an effective way and has a very wide surface area which can be cleaned more effectively by flashy compared to the conventional method.
Nanomaterial assault can remove a wide variety of pollutants it is a unique high surface feature, it can be used to successfully remove hazardous metal ions from water, as well as microbial pathogens and both organic and inorganic solutes.
Issues/Challenges
Nanotechnology has not contributed to any human health or environmental issues yet, but there are still worries about the potential for toxicity toward humans and the environment. Studies have shown that they can also be toxic because of some properties of nanomaterials that make them attractive such as shape, structure, size, and reactivity. However, the impact of nanomaterial on health and the environment is difficult to determine because the method and technique for such a task have not yet been well, developed.
Water Purification by Adsorption.
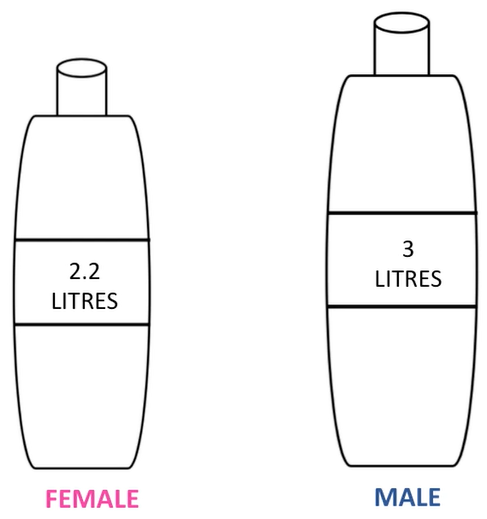
By adsorption, Clean purified water is an essential substance for all body. In order to stay healthy, a male and female should consume three litres and 2.2 litres of water a day respectively. If you drink less than this requirement, you will end up dehydrated. And if you don’t drink at all, then you might as well start digging your grave.
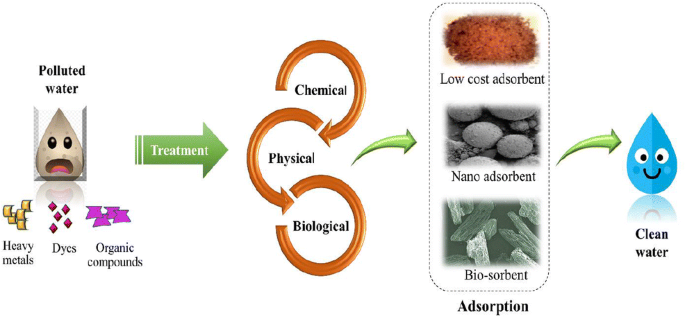
 I’ll explain how adsorption can be used to purify water in this section. When atoms, ions, or molecules from a gas, liquid, or dissolved solid adhere to a surface, the process is called adsorption.
I’ll explain how adsorption can be used to purify water in this section. When atoms, ions, or molecules from a gas, liquid, or dissolved solid adhere to a surface, the process is called adsorption.
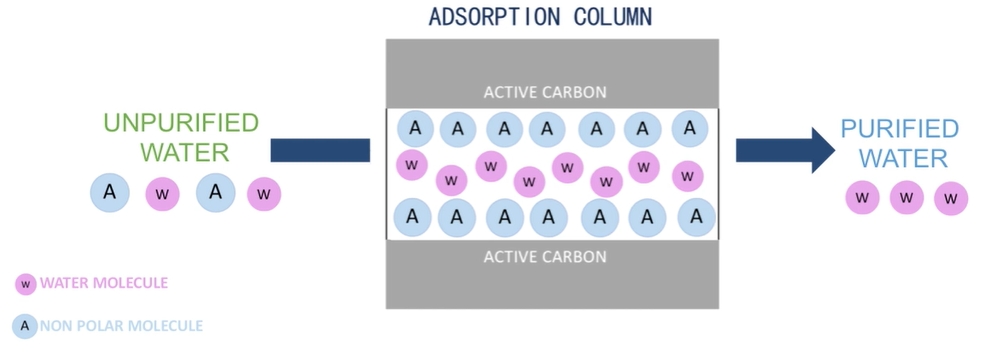 In the case of water purification water consisting of all its solutes passes through a column containing active carbon, active carbon is utilised as it has an incredibly large surface area per unit volume, making it suitable for this process. As the water flows through all the nonpolar less soluble in water particles stick to the surface of the carbon. Hence adsorbed water flowing out does not consist of these solutes. Hence purified organics such as unwanted tastes and smells are examples of what can be removed from the impurified water using this process.
In the case of water purification water consisting of all its solutes passes through a column containing active carbon, active carbon is utilised as it has an incredibly large surface area per unit volume, making it suitable for this process. As the water flows through all the nonpolar less soluble in water particles stick to the surface of the carbon. Hence adsorbed water flowing out does not consist of these solutes. Hence purified organics such as unwanted tastes and smells are examples of what can be removed from the impurified water using this process.
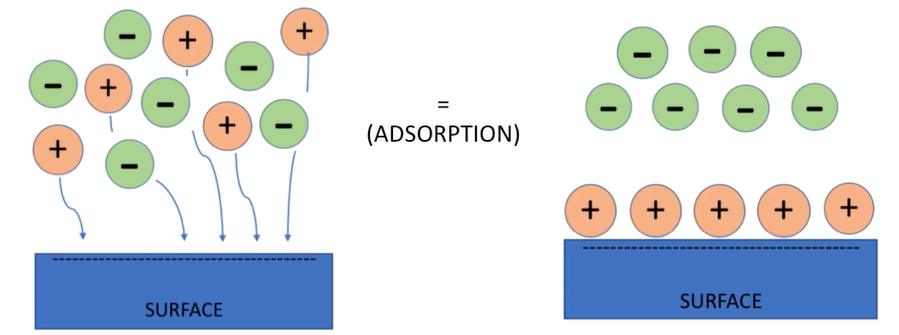 Activity level of adsorption is based on
Activity level of adsorption is based on
- Concentration
- Temperature
- Polarity of substance.
The highest rate of adsorption will be when there’s a high concentration of solids in water, a high temperature and most neutral solute particles. This was an introduction to watching prefiguration by adsorption.
The nanoparticle for water purification under study is nanobiotechnology. Water pollution that is any contaminant present in the water body that causes a harmful effect to a living organism that is called water pollution.
The water pollutants that cause pollution in the water may be dyes pesticides, organic pollutants, Poly aromatic hydrocarbons, heavy metals, these are some of the common examples, which create the problem, or which involved in the water pollution. Water pollution it can be point from the type according to the type it may be the point, or it may be the nonpoint source that is the source of water contaminant.
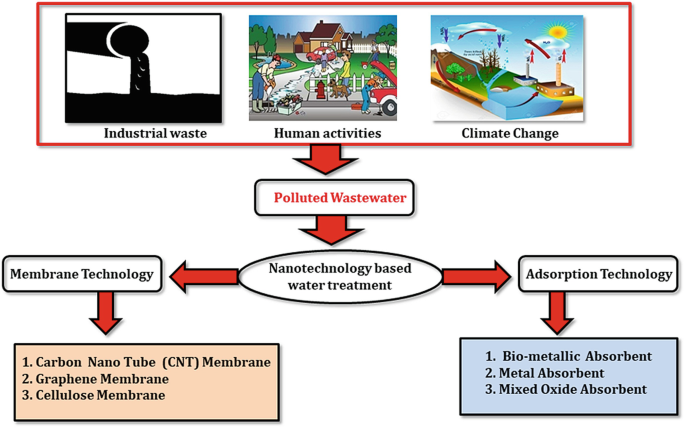 The points source is from that wastewater from the industries
The points source is from that wastewater from the industries- Second is non-point that is rainwater that is collect water from the different region
We know quality of drinking water; it is a necessity for the mankind and unfortunately or around about 1 billion people around the world they do not get the save water and among this 800 million people they belong to the that is from the rural area or from the village area.
It is also a big challenge to get a safe drinking water because it is a lot of that is problem is also due to the anthropogenic activity. The main source for water it is a from the pounds that is from the groundwater or lake these are the source that can be used for the agriculture as well as the domestic purpose and this is only the 4% of the total and 97% that is present in the ocean.
So, the problem is worldwide, and the contaminated sites are increasing day by day. Again, there are a lot of methods are available
- Physical
- Chemical,
- Biological methods
These are available for the treatment of this environmental or the water pollutant.
Disadvantages
But again, these have the limitation. Now, that first for example, biological method even has the limitation because it results in the formation of sludge in the scale that is in the secondary treatment process, because this process occurring mainly three step one is the primary, secondary treatment, then tertiary treatment.
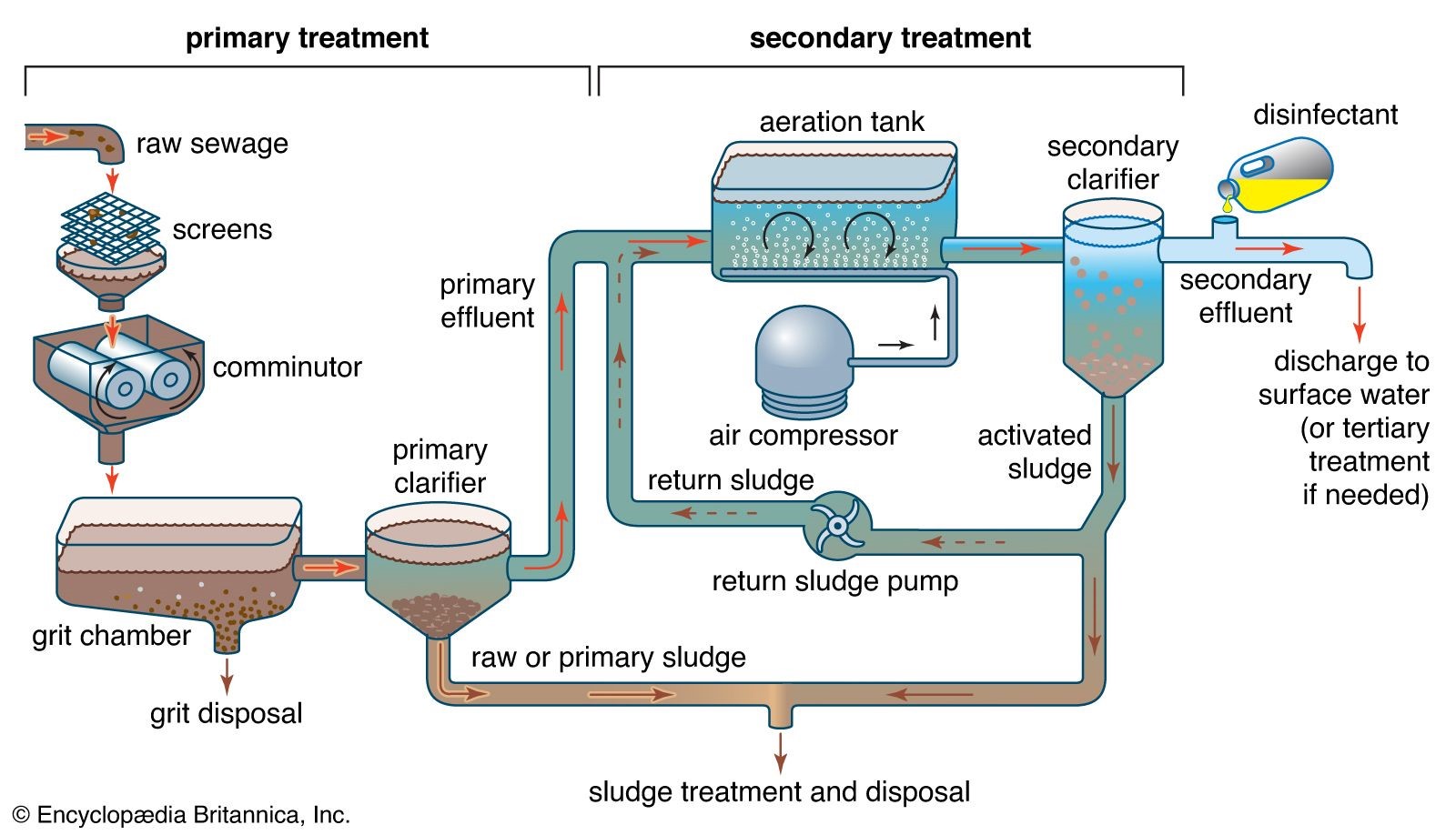 Primary it is a physical process where the sedimentation takes place, secondary where the biological and chemical method are involved. In tertiary treatment where chemical physical and biological methods are involved. Secondary biological method itself produced a large sludge and it is also created problem because we require the unfertile land for their dumping.
Primary it is a physical process where the sedimentation takes place, secondary where the biological and chemical method are involved. In tertiary treatment where chemical physical and biological methods are involved. Secondary biological method itself produced a large sludge and it is also created problem because we require the unfertile land for their dumping.
So, these also that we know the chemical and physical method they are not the cost-effective method and result in the formation of toxic substance and even these are also not a cost-effective method.
Nano Technology (Nanoparticle)
The nanoparticle because of their unique property, they have remarkable potential to remedy this water pollution. let us start with the main objective of this article or the blog is
- The first one is that you will understand the various nanoparticle used for the water pollutant revolution.
- Second, we will understand the mechanism of removal of this pollutant by using the nanoparticle
- Third one is they also understand the mechanism because carbon nanotubes (CNT) and titanium dioxide (TiO2) they are mainly used for the removal of this water pollutant.
We will understand the mechanism of removal of this by using these two nano particles and lastly, that is we will understand the application of this nanoparticle far the removal of this water pollutants.
 Advantages of Nanoparticles
Advantages of Nanoparticles
The various nanoparticle and they are used in the removal of environmental pollutant. Because nano particle they have the nano material, they have the unique property, high surface to volume ratio and this are responsible for their because they also have the photocatalysts mechanism and they have strong adsorption toward the pollutant and an answer redox mechanism also that is why they are mainly used to remedy the environmental pollutant.
Now, any remediation method for the pollutant it should meet the four conditions.
- The first one is that should be the cheapest method.
- Secondly, it should be the recycle of this a treatment agent
- Third one is it should be the cost effective also and there is nontoxic product formation.
 Which are mainly used for the remediation of this environmental pollutant, they are mainly according to their nature of mechanism or the action or according to their nature that is of three types
Which are mainly used for the remediation of this environmental pollutant, they are mainly according to their nature of mechanism or the action or according to their nature that is of three types
- One is nano adsorption. Here the nano particle they are dissolved the various environmental or the water pollutant.
- Second one is nano catalyst. For example, the best example is titanium dioxide which can act as a which show the mechanism of photo catalysts.
- Third one is nano membrane or nanofiltration. Reverse osmosis and nanofiltration are two examples of membrane processes where the water is passed through and treated using nanoparticles.
Nano Adsorption
As Nano adsorbents manufacturer the capability of the nano particle or the nano material to adsorb gases or the dissolved solids on the surface that is called Nano adsorption
Here that absorbs molecules they are called adsorbate and the nanomaterials they are called Nano adsorbent. It is just like the chromatography, or the column chromatography are the thin layer chromatography where the material that are adsorbed that is called adsorbate it and that is the column which adsorb on which the material is adsorb that are called adsorbent.
 But in this instance, the term “nano adsorbent” is utilized since nanomaterial is being used. Because of the nanomaterial’s tiny size and huge surface area, additional adsorption of organic and heavy metal contaminants on their surfaces is made possible and like where the chromatography thin layer chromatography, the adsorption because the principle is adsorption and partitioning here also therefore, it will adsorption will depend upon the partitioning coefficient as well as the adsorption coefficient of the pollutant on the surface of this nanoparticles.
But in this instance, the term “nano adsorbent” is utilized since nanomaterial is being used. Because of the nanomaterial’s tiny size and huge surface area, additional adsorption of organic and heavy metal contaminants on their surfaces is made possible and like where the chromatography thin layer chromatography, the adsorption because the principle is adsorption and partitioning here also therefore, it will adsorption will depend upon the partitioning coefficient as well as the adsorption coefficient of the pollutant on the surface of this nanoparticles.
Nano particles and their uses in the removal of water contamination
As Nano-adsorbents for wastewater treatment plant manufacturer, the most commonly used nanoparticle which are used, or which show the phenomenon Nano adsorption they are carbon nanotubes (CNT), dendrimers and oxide-based nanoparticles.
 Carbon Nanotubes (CNT) adsorbent
Carbon Nanotubes (CNT) adsorbent
CNT as a nano adsorbent they are widely studied area or the material that have the capability to remove heavy metals dyes as well as the organic pollutant like pesticide residues from the wastewater through the adoption phenomena or through the adoption process.
 It shows the high adsorption capacity as compared to the conventional granular and powdered activated carbon technique which are used to remove the pollutant and it also involves the chemical interaction what is the mechanism behind the adsorption of polar and nonpolar compounds that is it involves chemical interaction for polar compounds and physical interaction far the nonpolar compounds and CNT surface they are highly hydrophobic in nature and in the aqueous solution or in the aqueous form, they aggregate and aggregate to form the four probable site for the adoption of different water contaminants.
It shows the high adsorption capacity as compared to the conventional granular and powdered activated carbon technique which are used to remove the pollutant and it also involves the chemical interaction what is the mechanism behind the adsorption of polar and nonpolar compounds that is it involves chemical interaction for polar compounds and physical interaction far the nonpolar compounds and CNT surface they are highly hydrophobic in nature and in the aqueous solution or in the aqueous form, they aggregate and aggregate to form the four probable site for the adoption of different water contaminants.
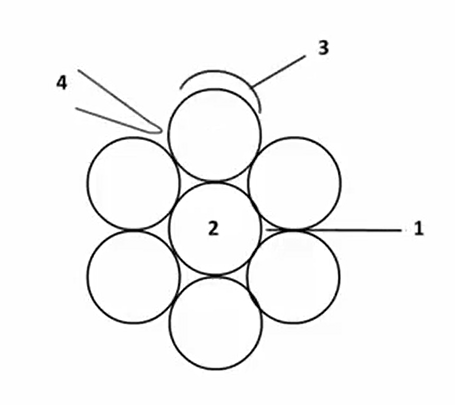
 These sites are
These sites are
- Internal sites
- Interstitial sites or channels
- Outer surface and
- External grooves
As shown in the figure that is one that is the interest internal side second one is the interstitial channel and third one is the outer that is outside surface fourth one is external group.
Now, the question arises where the maximum adsorption occurred maximum adsorption that is occurred in the outer surface and grows because they are in direct contact with the pollutant or that contaminant or the absorbing material.
 So, adsorption equally then it rates more rapidly on the external side that is outer surface and grooves because they are in direct contact with the absorbing material. So, the overall adsorption activity of CNT it increases by that surface modification, very important phenomena by modifying or by functionalization that is with both covalent and noncovalent functional functionalization as well as modification, we can increase and also researchers try to increase the adsorption activity of this carbon nanotubes.
So, adsorption equally then it rates more rapidly on the external side that is outer surface and grooves because they are in direct contact with the absorbing material. So, the overall adsorption activity of CNT it increases by that surface modification, very important phenomena by modifying or by functionalization that is with both covalent and noncovalent functional functionalization as well as modification, we can increase and also researchers try to increase the adsorption activity of this carbon nanotubes.
Covalent functionalization
Covalent functionalization that is off CNT It is mainly of two types
- One is defect and
- Second one is sidewall modification
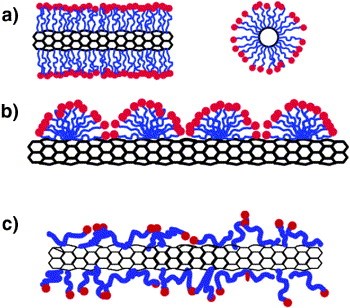 In the defect modification that is a functional group they are because they are a test to increase the interaction with the contaminant further removal of that particular contaminant or pollutant. Functional group they are present at the end of CNTs carbon nanotubes, and they have capability to bond with other functional groups.
In the defect modification that is a functional group they are because they are a test to increase the interaction with the contaminant further removal of that particular contaminant or pollutant. Functional group they are present at the end of CNTs carbon nanotubes, and they have capability to bond with other functional groups.
In the sidewall as the name indicates, there is a covalent bonding of functional group on the sidewall of the carbon nanotubes, as we have already discussed that carbon nanotubes, they have wall like structure that is a single wall nano carbon nanotube, multiwalled nanotubes that is why the sidewall if sidewall or they are involved in the covalent bonding of functional group then it is called sidewall modification.
Noncovalent functionalization does not change the physical property of this carbon nanotubes, because there is no covalent bond formation occurred and also, they keep the structure intrinsic SP2 hybridization of orbital that remain unchanged because it is a non-covalent interaction.
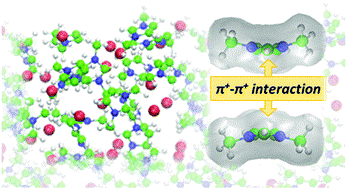
It mainly involved up Pi to Pi interaction between the conjugated molecule and the sidewall of the carbon nano tubes that is group present on the sidewall of the carbon nanotubes.
 Second method of non-covalent modification or functionalization it involves noncovalent hydrophobic interaction with the surface of Carbon Nanotube in aqueous solution that reduces the hydrophobic interface between the carbon nanotubes and their polar environment also. This is the diagram which showing both Pi to Pi interaction that is a non-covalent functionalization between the sidewall of the carbon nanotubes and that contaminant or other functional group which are attached to increase the adsorption capacity of the carbon nanotubes.
Second method of non-covalent modification or functionalization it involves noncovalent hydrophobic interaction with the surface of Carbon Nanotube in aqueous solution that reduces the hydrophobic interface between the carbon nanotubes and their polar environment also. This is the diagram which showing both Pi to Pi interaction that is a non-covalent functionalization between the sidewall of the carbon nanotubes and that contaminant or other functional group which are attached to increase the adsorption capacity of the carbon nanotubes.
Similarly, hydrogen bonding is also involved with the functional group, electrostatic group are the interaction are also involved if the group carry any charge the molecule or the charge functional molecule.
This diagram also shows that covalent as well as the non-covalent that is Pi to Pi interaction as shown in the red for that is a red functional group that is there is a Pi-to-Pi interaction of functional group with the sidewall of the carbon nanotubes. In that covalent modification. There is a covalent interaction between the functional group of this Carbon Nanotubes with the that is a molecule or the functional group that are attached to increase the adsorption capacity.
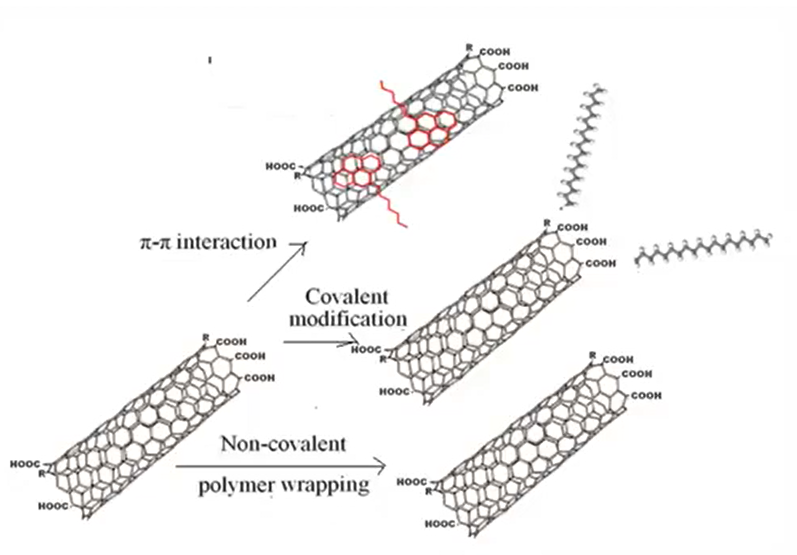 Other method of the surface modification of this carbon nanotube, they include the acid treatment and that is a mainly used treatment method is acid treatment, metal or the chemical functionalization it is a chemical functionalization It is mainly involved, but metal that is incorporation of this metal that is not a commonly used method and this acid statement, it always required different kinds of acid like sulfuric acid, hydrogen chloride, then there is a nitric acid, hydrogen chloride.
Other method of the surface modification of this carbon nanotube, they include the acid treatment and that is a mainly used treatment method is acid treatment, metal or the chemical functionalization it is a chemical functionalization It is mainly involved, but metal that is incorporation of this metal that is not a commonly used method and this acid statement, it always required different kinds of acid like sulfuric acid, hydrogen chloride, then there is a nitric acid, hydrogen chloride.
This acid not only remove the impurities, but also that is present on the carbon nanotubes, but also result in the chemical modification because it exposed the sight of this carbon nanotubes. So, there is a because it also provides some type of charges that is why the adsorption capacity, or the adsorption is increases when the contaminant it become in contact with this carbon nanotubes.
Heavy metal adsorption on the CNTs
The adsorption of heavy metals onto carbon nanotubes comes next. This adsorption capability of carbon nanotubes toward heavy metals and it is also effective or a large range of pH values, and it is mostly reliant upon the value of pH. Heavy metal ion adsorption on CNT carbon nanotubes does not have a specific mechanism that they are primarily involved in.
- The chemical interaction
- Physical adsorption
- Electrostatic attraction that is between heavy metal ion and the functional group present on the CNT surface or the carbon nanotubes surface
 The factor that affecting the adoption of these heavy metal ion they are
The factor that affecting the adoption of these heavy metal ion they are
- The surface charges
- Ionic form of heavy metal ions, whether it is present in the single valent ions, double valent ions, or the triple valent ions in the solution
- The experimental parameters, including temperature, contact time, and the starting metal ion concentration in the carbon nanotube mass.
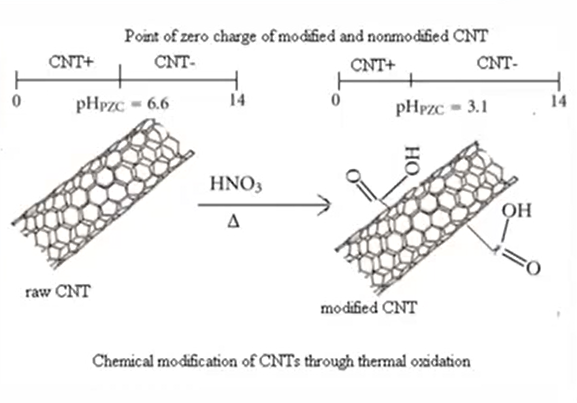
Point of zero charge
zeroth possible charge Due to the fact that pH is a key factor in the heavy metal’s adsorption on the surface of CNT, its significance in this process cannot be overstated. Point to zero charge is a pH level at which the charge density only exists on the surface of carbon nanotubes. It is a crucial factor in the adsorption of pollutants onto the surface of carbon nanotubes.
Substrate in aquas solution that have higher point to zero charge ratio or the value in that case the negative surface charge some that deprotonation they favour for the adsorbing cat ions through the electrostatic interaction the adsorption of cat ions is it decreases with decreases is the pH due to the neutralization of surface charge in contrast is surface placed in the solution that is having lower then P point to charge ratio the positive surface that is from the protonation.
 They favour for that adsorbing anion through the electrostatic interactions that is shown in the figure also where CNT that is a raw carbon nanotube where the point to charge ratio is 6.6 and second one is the modified that is chemically modified where the point to charge ratio become 3.1 as shown in the figure that it as the point to charge ratio increases or decreases it favours the negative surface charge as shown in the diagram. It favours the adsorbing of cat ions and the positive surface favours the absorbing of an ions.
They favour for that adsorbing anion through the electrostatic interactions that is shown in the figure also where CNT that is a raw carbon nanotube where the point to charge ratio is 6.6 and second one is the modified that is chemically modified where the point to charge ratio become 3.1 as shown in the figure that it as the point to charge ratio increases or decreases it favours the negative surface charge as shown in the diagram. It favours the adsorbing of cat ions and the positive surface favours the absorbing of an ions.
 Other organic pollutant removal by using CNT. carbon nanotubes they have the capability to remove the organic pollutant as well as the heavy metals.
Other organic pollutant removal by using CNT. carbon nanotubes they have the capability to remove the organic pollutant as well as the heavy metals.
How they remove this heavy metal organic pollutant and because of their characteristic property, nano size and also because of their various modification functional modification and which allows the adsorption of this organic pollutant.
Now, the various interaction which are involved in this removal, these are the Pi-to-pi interaction, we have already discussed in the previous then the hydrogen bonding ionic interaction because when ions or the ionic groups are present on the functional group, they attract another ionic group.
So, there is also an Ionic interaction for the removal of this organic nutrient. So, CNT are the carbon nanotubes they are most commonly used for the remediation of water pollutant and the mechanism is of their adsorption that is their structure then that may be the multiwalled carbon nanotubes or maybe the single walled carbon nano tubes.
 Dendrimer Nano Adsorbent
Dendrimer Nano Adsorbent
Next nano adsorbent that are used is a Dendrimer nanoparticle which are also used as a nano absorbent like CNT it is used for removing organic pollutants as well as heavy metals like carbon nanotubes. Heavy metal they are adsorbed by the tailored exterior branches on dendrimer whereas, organic compounds they are mainly absorbed by the anterior hydrophobic shells as shown in the figure.
 There is a surface group as well as the branches we have already discussed the structure of dendrimer in the previous module. So, an exterior surface with functional group, they are mainly involved in the removal of organic compounds, whereas interior branches they are involved in the removal of that is it other that is the organic compound, but the tailored or the exterior surface with functional surface group they are involved in the removal of heavy metals.
There is a surface group as well as the branches we have already discussed the structure of dendrimer in the previous module. So, an exterior surface with functional group, they are mainly involved in the removal of organic compounds, whereas interior branches they are involved in the removal of that is it other that is the organic compound, but the tailored or the exterior surface with functional surface group they are involved in the removal of heavy metals.
Currently, the assessor uses Dendrimer conjugated magnetic nanoparticles for the removal of these heavy metals. For instance, when an amine, such as poly Amido amine (PAMAM), is ended, Dendrimer has a high affinity for attaching metal ions to their surface by coordination with the amine.
Oxide Based Nanoparticle
Next is the oxide-based nanoparticle which are used as a nano adsorbent example are zinc oxide that is a zinc oxide nanoparticle, magnesium oxide nanoparticle. These are mainly used for the removal of organic pollutant as well as heavy metal.
 Next is the single iron where the Fe, Fe304 and binary Fe/Fe3O4 nanoparticle they are mainly used for the removal of heavy metal from the water that is due to the oxidation state of this iron particle or the iron element. Because in the aqueous solution, this iron form changes to undergo oxidation to change ferrous than ferric. And then they further reduce the organic nutrients that come in contact with this iron element or the iron oxide element.
Next is the single iron where the Fe, Fe304 and binary Fe/Fe3O4 nanoparticle they are mainly used for the removal of heavy metal from the water that is due to the oxidation state of this iron particle or the iron element. Because in the aqueous solution, this iron form changes to undergo oxidation to change ferrous than ferric. And then they further reduce the organic nutrients that come in contact with this iron element or the iron oxide element.
Nano Catalyst
Nano catalyst involved
- The photo catalyst,
- Electro catalysts and
- Fenton based catalyst
But photocatalyst It is mainly used in further remedy action of pollutant, and we have already discussed that a photocatalysts activity it is mainly shown by the titanium dioxide (TiO2) that is a nanoparticle which your accident photocatalysts phenomena that is in the presence of light they can act as a catalyst and photocatalysts it is defined as catalytic reaction, which lead to the production of catalyst by adsorption of light.
The majority of common photocatalyst are the sulphide semiconductors are the metal oxide like titanium dioxide (TiO2) and zinc oxide (ZnO) they are the excellent example of photocatalytic nanoparticle.
But for the removal of these organic pollutants or heavy metals or any other pollutant or contaminant titanium dioxide are mainly used because of their excellent photo catalytic activity.
Photo Catalysis by The Titanium Dioxide (TiO2)
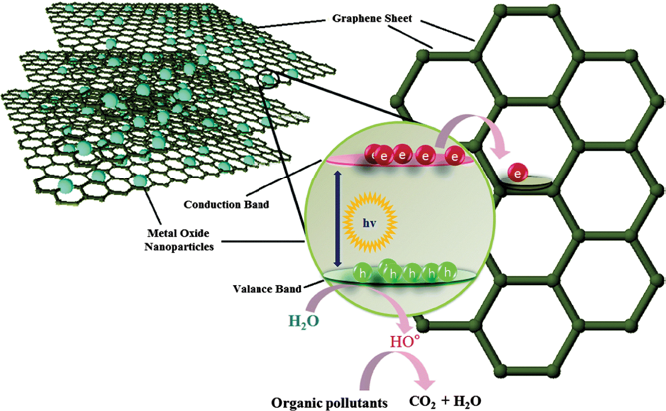
Photocatalytic tape meant using titanium dioxide it is a well-known advanced oxidation process, where the overview of mechanism is that when light absorbed titanium dioxide surface electron are released at this electrode released, they combined with the oxygen molecule to form the superoxide ion, these superoxide ion due to the release of electron the surface of the titanium dioxide become positive charge that ishole are created and they take electron from the moisture of air or from the moisture their loss electron and become hydroxide ion.
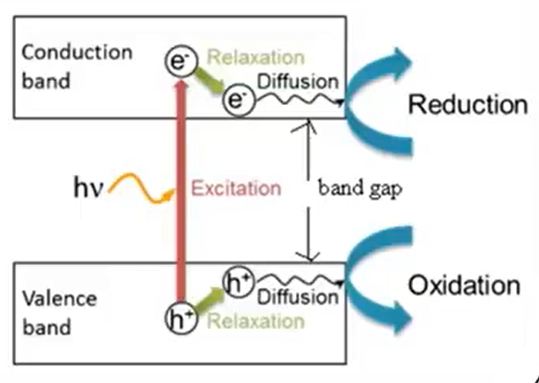 Thus, this concludes the basic description of the mechanism. Due to its proper valence band and conduction band positions, titanium dioxide is a semiconductor that is mostly used for the photocatalytic removal of pollutants from water.
Thus, this concludes the basic description of the mechanism. Due to its proper valence band and conduction band positions, titanium dioxide is a semiconductor that is mostly used for the photocatalytic removal of pollutants from water.
It mostly happened as a result of a series of hydroxylation reactions that the hydroxyl radical started. When titanium dioxide is illuminated by a photon with energy greater than or equal to the valence band and the conduction band, the lone pair or the lone electron will be photoexcited to the empty conduction band. This citation of this nanomaterial by a photon result in the production of free electron and hole in the titanium dioxide photocatalyst, and this charged particle they drift to the surface and then interact with t.
Since holes are positive charges, they interact with water molecules to produce radical plus hydrogen ions, whereas electrons directly interact with dissolved oxygen to produce reactive oxygen species like superoxide ions, which degrade the pollutant present near the or present near c. As a result, electrons are primarily involved in reduction reactions, as shown in the figure, while holes are involved in oxidation reactions.
So, there are two main mechanisms as shown in the figure
- One is the reactive oxygen species production. They’re involved in the degradation of pollutant and
- Second one is the peroxide radical that actually damage the cell.
 The peroxide radical and superoxide radical are the reactive oxygen species that are produced; they decay to pollutants and interact with water molecules to produce the peroxide radical and hydroxide ion.
The peroxide radical and superoxide radical are the reactive oxygen species that are produced; they decay to pollutants and interact with water molecules to produce the peroxide radical and hydroxide ion.
These generated peroxide radicals then combine with the hydrogen ion to form the hydroxide ion and radical. Therefore, the hydroxide ions in their whole were oxidised to create the hydroxide radical, which went on to harm both the gramme positive and gramme negative bacteria’s structure and function.
Therefore, the pH of two solutions—one of which is the degraded solution and the other is the pH of the catalytic solution created during the preparation of this catalyst—is the primary parameter that can affect the photocatalytic process. The second primary parameter that can affect the photocatalytic process is the oxidizing agent.
Nano membrane filtration
The final is the nano membrane filtration nano membrane, which is mostly used to remove organic pollutants but also has the ability to remove heavy metals. Furthermore, the majority of them are made of either polymers, nanofibers, nanopore membranes, or polymer nanolayers.
 Functionalized Magnetic Nano-Adsorbent for Efficient Wastewater Treatment for Coronavirus Pandemic
Functionalized Magnetic Nano-Adsorbent for Efficient Wastewater Treatment for Coronavirus Pandemic
The front line for Mag Zorb is COVID-19. The Chinese government alerted the globe that the virus was spreading rapidly in those villages in December 2019. In the months that follow, it spreads to neighbouring nations, and instances quickly double. Everyone in Korea simply referred to the virus as the severe acute respiratory syndrome linked Coronavirus.
 Since there is now no vaccination available for the coronavirus, we must socially modify our behaviour to function as a social vaccine. Two things are clear from this.
Since there is now no vaccination available for the coronavirus, we must socially modify our behaviour to function as a social vaccine. Two things are clear from this.
- The first one is not getting infected.
- Secondly, not infecting others.
Even though it may seem insignificant, washing our hands frequently and maintaining cleanliness and hygiene are the best things we can do. Therefore, during this pandemic, there is a huge need for a steady and constant supply of clean water.
 Nonetheless, as demand is spiking for washing hands frequently and cleaning everything, the access of clean water becomes a challenge faced by several countries including many countries during COVID 19 pandemic. Therefore, there is an increased concern to treat generated wastewater to ensure that there is sufficient clean water supply for communities and societies.
Nonetheless, as demand is spiking for washing hands frequently and cleaning everything, the access of clean water becomes a challenge faced by several countries including many countries during COVID 19 pandemic. Therefore, there is an increased concern to treat generated wastewater to ensure that there is sufficient clean water supply for communities and societies.
Recently, many countries had faced with water shortage due to water pollution scandal happened. Consequently, over 1.2 million people who are affected with water disruption could not receive safe water supply sufficiently. In these regards, we as Nano-adsorbents for water treatment manufacturer, have an effective solution by introducing the usage of new adsorbent called mag Sorb.
 This mag sorb is specialised for a wastewater treatment, it is an ideal and economical adsorbent for treating pollutants effectively thus able to provide safe and clean water to community. The mag sorb is made from agricultural waste-based product which is low cost and environmentally friendly. As Nano adsorbents for water treatment manufacturer, the preparation process of mag Sorb is simple with no sophisticated equipment’s or chemicals required.
This mag sorb is specialised for a wastewater treatment, it is an ideal and economical adsorbent for treating pollutants effectively thus able to provide safe and clean water to community. The mag sorb is made from agricultural waste-based product which is low cost and environmentally friendly. As Nano adsorbents for water treatment manufacturer, the preparation process of mag Sorb is simple with no sophisticated equipment’s or chemicals required.
Benefits of mag sorbets
In addition, it is easy for recovery and can be used for several times with outstanding performance remained. The benefits of mag sorbets it is versatile, simple to prepare, and it can easily be separated from the wastewater to be reused for a few times. in these regards, it could combat pollution in wastewater, thus solve water supply issues that are being facing by several places.
As Nano-adsorbents Manufacturer, it can be seen that the mag Sorb offer more benefits compared to commercial activated carbon in term of high yield and production mild operating condition and superior adsorption performance for water treatment. Besides that, the mag sorb meets with market needs such as
- Low cost,
- Simple preparation process,
- Eco friendly and
- Reusable with excellent performance.
These good properties of mag solid would bring a lot of benefits to wastewater treatment industries, especially during outbreak COVID-19. The uniqueness or strengths of our product is its cost effectiveness associated with simple preparation process. The usage of agricultural wastes are by-product materials, easy for recovery and able to be reused for several times.
Limitations or Weaknesses
However, there are some limitations or weaknesses for achieving the objectives such as the lack of awareness among prospective customers and the absence of sales expertise, these limitations may become a challenge to our business to be able to perform at optimum level.
During this COVID 19 pandemic. There are high in demand of safe and clean water supplies and sanitation and hygiene is the most important roles to control the spreading of virus. Thus, various industries of wastewater treatment could be potential target market subsequently leads for rapid growth of the business.
4-How Photocatalysis works with TiO2
The principles and mechanism of photocatalysis will be illustrated in this section. An intense power of breakdown is produced on the titanium dioxide-coated surface when light energy from the sun or other fluorescent lighting is radiated onto titanium dioxide.
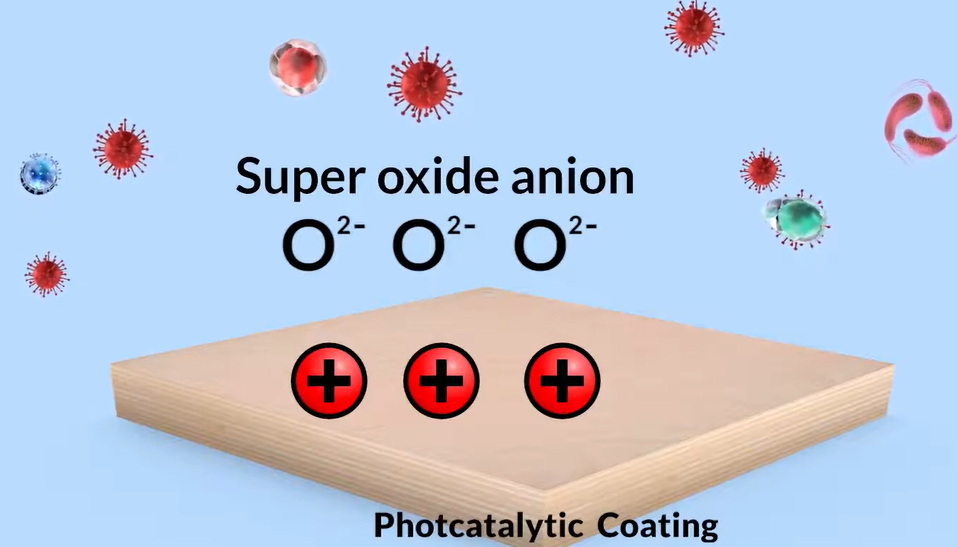 Microorganisms and viruses are repeatedly destroyed till they are harmless due to its characteristics. Effective photo catalysis lasts for practically a lifetime. Let’s examine how these functions. Titanium dioxide emits electrons when light energy is incident on its surface. The oxygen and released electrons combine to form superoxide anion.
Microorganisms and viruses are repeatedly destroyed till they are harmless due to its characteristics. Effective photo catalysis lasts for practically a lifetime. Let’s examine how these functions. Titanium dioxide emits electrons when light energy is incident on its surface. The oxygen and released electrons combine to form superoxide anion.
Titanium dioxide’s surface develops a positive charge and absorbs electrons from the air’s moisture. When air moisture loses electrons, it changes into a hydroxyl radical. The superoxide anion and hydroxyl radicals by their power of oxidation destruction decompose organic compounds such as COVID-19 unwanted bacteria, fungus, salmonella, and offensive odours, turning them into water or other harmless substances and dispersing them into the atmosphere. The hydroxyl radical is frequently referred to as the detergent of the troposphere because it reacts with many pollutants, decomposing them through cracking and frequently acting as the first step to their removal.
 5-Graphene Filtration (A revolution in Desalination technology)
5-Graphene Filtration (A revolution in Desalination technology)
They made a significant advancement in the graphene-based desalination method by successfully removing 97 percent of common salts while using minimal energy. The capital costs of desalination plants are substantial, and the existing reverse osmosis desalination technology is energy intensive.
This finding is crucial since by 2025, 14% of the world’s population would be affected by water scarcity. In addition, filtration technology based on graphene may soon be available in your kitchen. Let’s first examine the current RO desalination method before moving on to graphene-based desalination technology.
 Take into account this intriguing experiment. Using a semi-permeable membrane, salty water and pure water are separated. A semipermeable barrier will let the passage of water molecules but not salt ions because nature constantly strives to maintain a balance in the concentration of molecules. The seawater level will therefore naturally rise if you wait a few minutes.
Take into account this intriguing experiment. Using a semi-permeable membrane, salty water and pure water are separated. A semipermeable barrier will let the passage of water molecules but not salt ions because nature constantly strives to maintain a balance in the concentration of molecules. The seawater level will therefore naturally rise if you wait a few minutes.
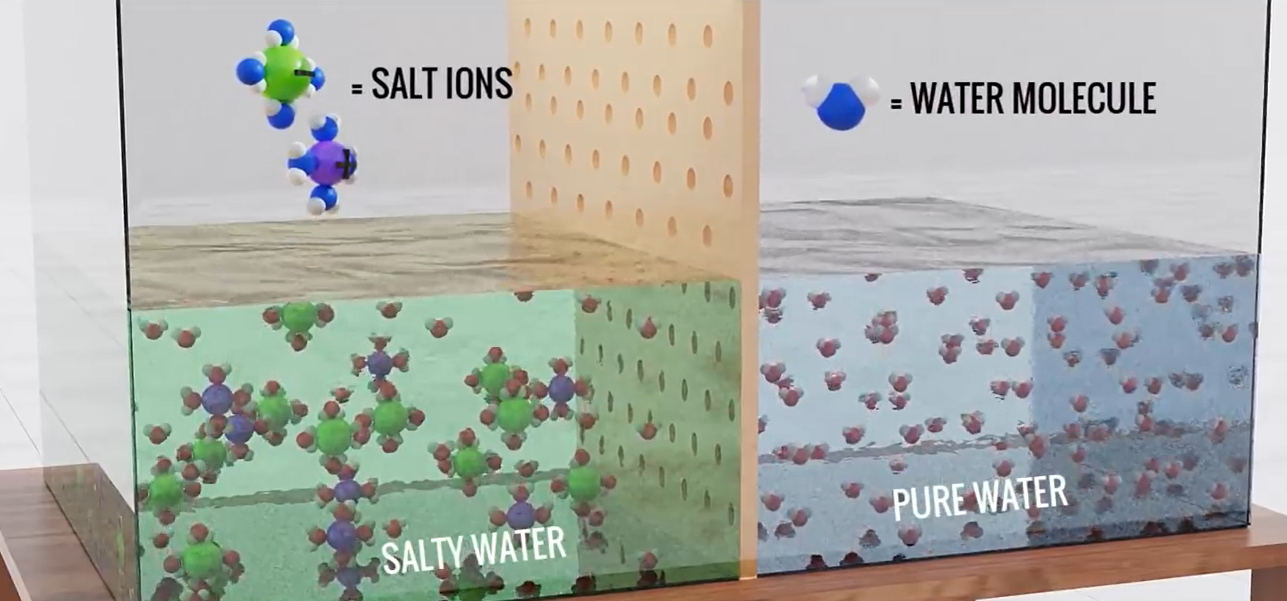 Here the water molecules from the right side migrate to the left side in an attempt to balance the salt level concentration. This naturally occurring process is called osmosis. We definitely don’t want this result. What we need is the exact reverse of this process, water molecules from the left side should migrate to the right side.
Here the water molecules from the right side migrate to the left side in an attempt to balance the salt level concentration. This naturally occurring process is called osmosis. We definitely don’t want this result. What we need is the exact reverse of this process, water molecules from the left side should migrate to the right side.
So, what can we do to get pure water we can apply good pressure on the saltwater side and the increased pressure will force the water molecules in the salty side to migrate to the clean water side. Obviously, the semipermeable membrane blocks migration of salt ions. This method is known as reverse osmosis.
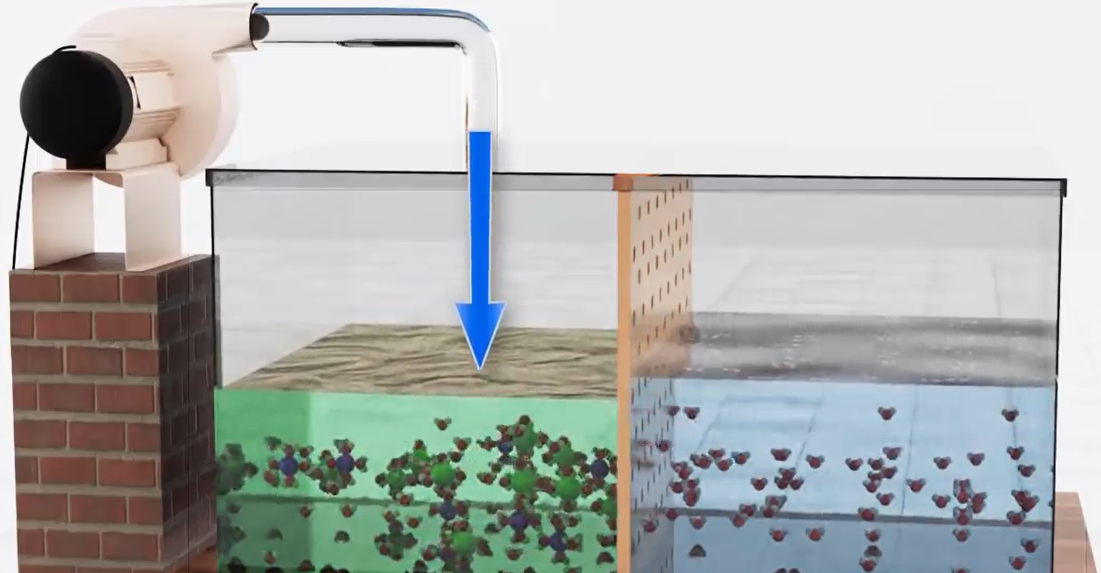 However, pumps require enormous amounts of energy to apply pressure on water and the equipment costs make it difficult to build more desalination plants. The quantity of pure water is smaller as well.
However, pumps require enormous amounts of energy to apply pressure on water and the equipment costs make it difficult to build more desalination plants. The quantity of pure water is smaller as well.
Graphene Nano particles
Now we’ll introduce to your graphene. Graphene is a wonder material with a lot of miraculous properties. Let’s see how we can use it for the desalination process. A graphene sheet with a lot of small pores is an excellent candidate for the desalination process. A graphene sheet can be made with the desired pores by using ion bombardment and selective etching techniques.
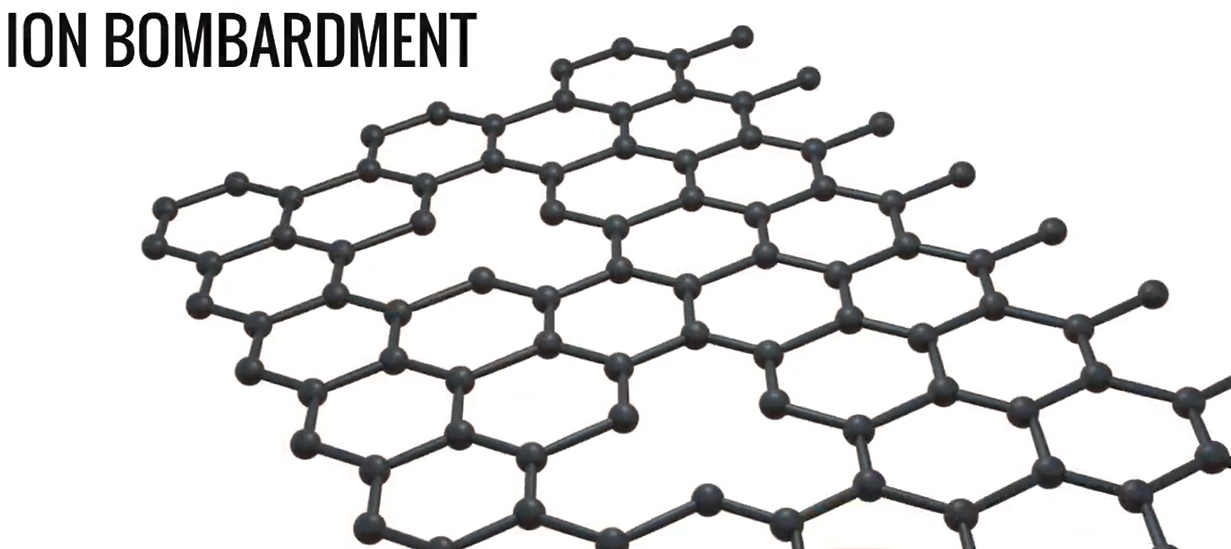 The salt ions are blocked yet the water molecules can simply pass through these pores. However, the location of the holes cannot be predicted exactly on an industrial scale. Therefore, using graphene as a filtration membrane for mass production is impractical. Graphene oxide, which is created by oxidising graphite, is a good substitute for graphene. Later, to obtain graphene oxide, these layers are exfoliated. The production of sheets made of graphene oxide is simple and inexpensive.
The salt ions are blocked yet the water molecules can simply pass through these pores. However, the location of the holes cannot be predicted exactly on an industrial scale. Therefore, using graphene as a filtration membrane for mass production is impractical. Graphene oxide, which is created by oxidising graphite, is a good substitute for graphene. Later, to obtain graphene oxide, these layers are exfoliated. The production of sheets made of graphene oxide is simple and inexpensive.
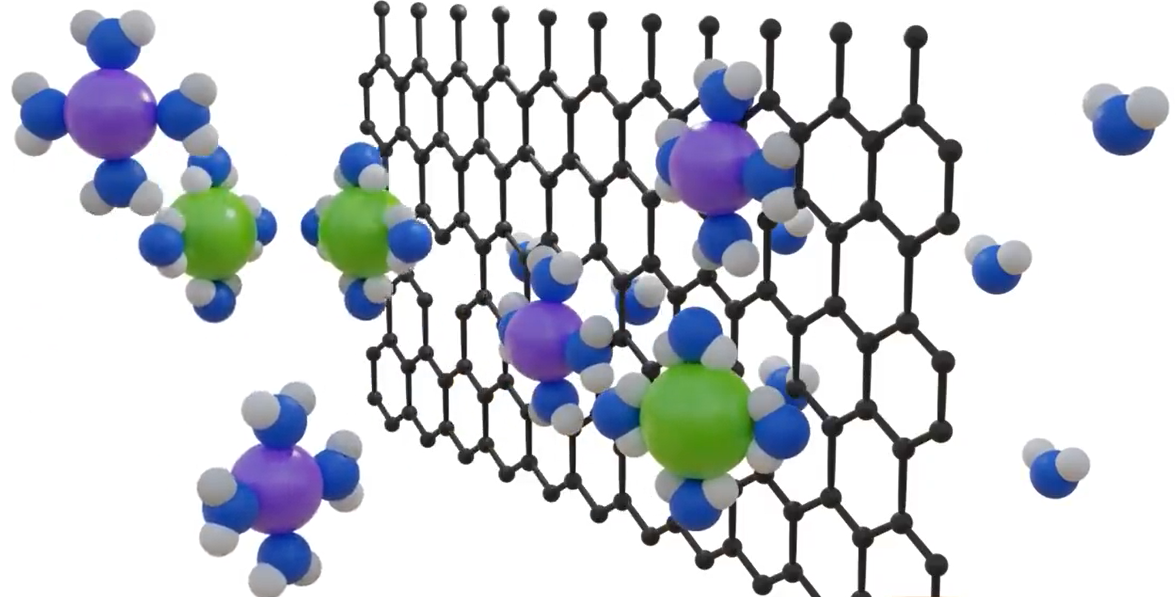 The hydrophilic nature of graphene oxide, which results in rapid water flow through it, is an intriguing characteristic that makes it perfect for desalination. But using ion bombardment to create pores on GO sheets is not a workable option. By stacking GO sheets and adjusting the space between them, pores can also be made.
The hydrophilic nature of graphene oxide, which results in rapid water flow through it, is an intriguing characteristic that makes it perfect for desalination. But using ion bombardment to create pores on GO sheets is not a workable option. By stacking GO sheets and adjusting the space between them, pores can also be made.
Let’s put this recently created GO membrane to the test for our desalination needs. By means of capillary action, the water automatically travels through the membrane. The observed results indicate that there is no salt permeability at the 6.4 angstrom pore size. Salts are blocked because salt atoms have a greater width than water atoms. Seawater contains salt in an ionic form as Na+ and Cl -. When these salt ions are in water, they hydrate, but because they are charged, they draw water into concentric shells around them, increasing the diameter of the salt ions.
In order to inhibit these hydrated ions, the pore size of graphene oxide membranes is tailored appropriately. Covalent bonds, which are more powerful than hydrogen bonds between water molecules, are used to bind salt to water molecules. Because of this disparity in tensile strength, water molecules are easily broken and penetrated.
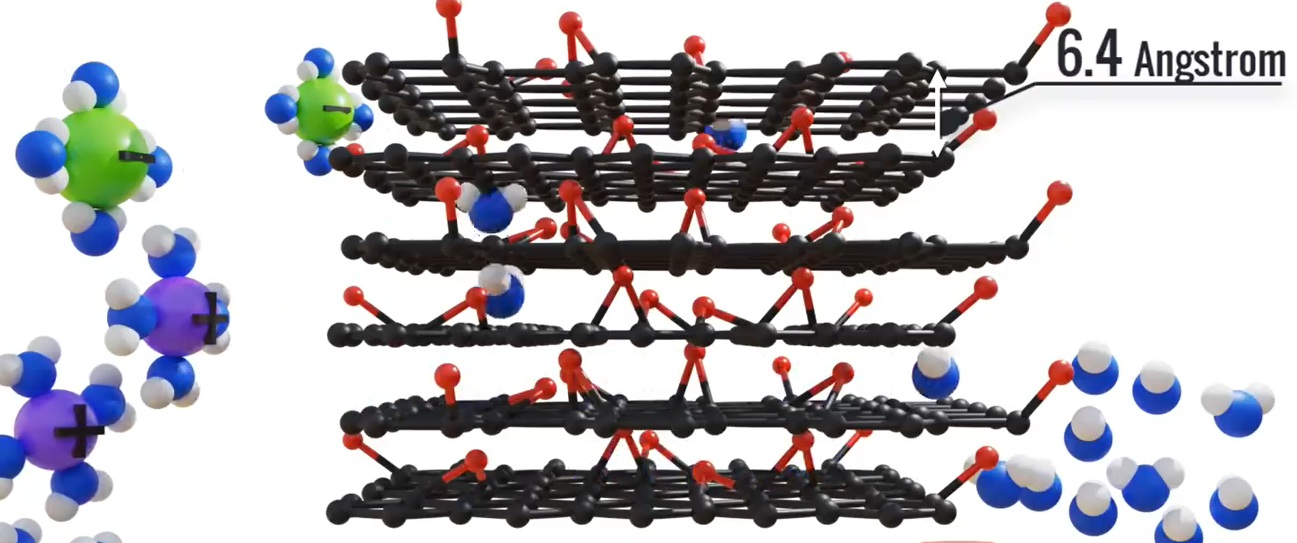 Salt to water bonds, however, are more difficult to break and barely pass through a GO membrane. Compared to graphene membranes, GO membranes are simpler to manufacture on a bigger scale.
Salt to water bonds, however, are more difficult to break and barely pass through a GO membrane. Compared to graphene membranes, GO membranes are simpler to manufacture on a bigger scale.
The design of a GO filtration mechanism is not over yet. If we use this GO stack for desalination, we won’t get good results because graphene oxide has higher affinity to water. When the stack of GO sheets is immersed in water, two to three layers of water molecules intercalate between pores causing it to swell.
 This swelling increases the pore size which can then permeate the salt atoms to overcome this problem. Stage cast epoxy is used to physically restrain the swelling of graphene oxide laminates. To create filtering membranes, layers of sky cast epoxy and graphene oxide laminate are alternated.
This swelling increases the pore size which can then permeate the salt atoms to overcome this problem. Stage cast epoxy is used to physically restrain the swelling of graphene oxide laminates. To create filtering membranes, layers of sky cast epoxy and graphene oxide laminate are alternated.
Advantages
Up to 90% to 95% of salts can be removed using a standard RO membrane. The graphene oxide-based membranes, however, are capable of removing up to 97 percent of salts. Due to their high tensile strength and greater affinity for water, graphene oxide membranes are ideal candidates for desalination.
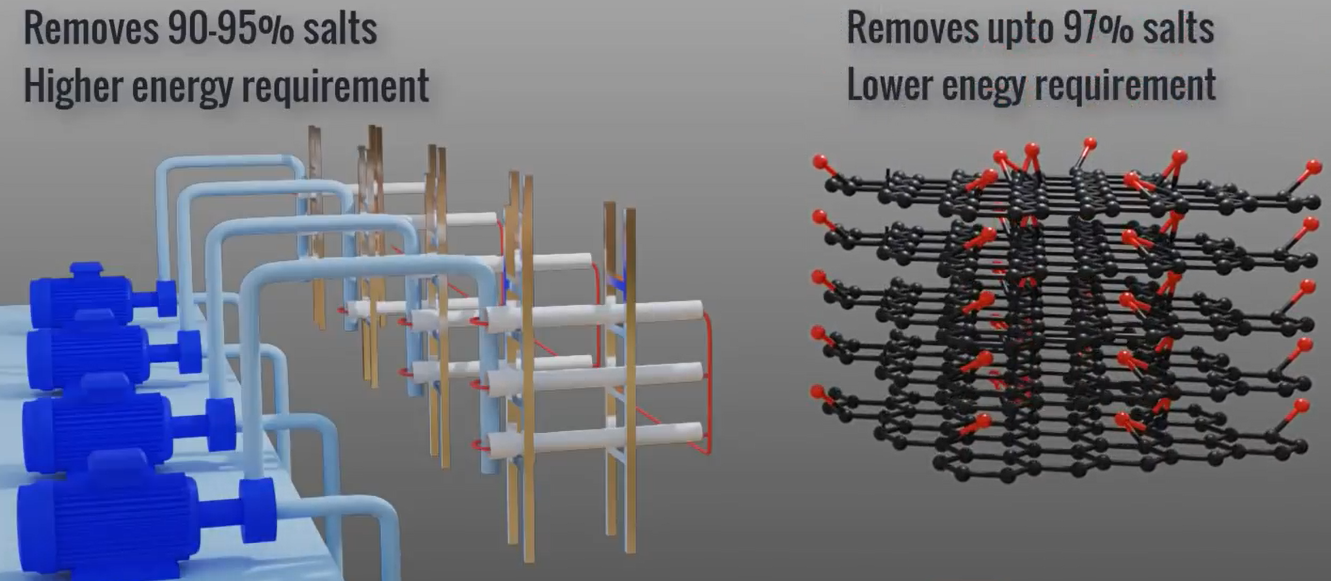 In RO plants, we need to reverse a naturally occurring process using external energy. Therefore, electrical energy required for the pump’s accounts for approximately 44% of an RO plants total cost. On the other hand, the graphene oxide approach uses a filtering procedure that occurs naturally. Using graphene sheets and graphene oxide membranes together is another innovation that could simplify manufacturing.
In RO plants, we need to reverse a naturally occurring process using external energy. Therefore, electrical energy required for the pump’s accounts for approximately 44% of an RO plants total cost. On the other hand, the graphene oxide approach uses a filtering procedure that occurs naturally. Using graphene sheets and graphene oxide membranes together is another innovation that could simplify manufacturing.
This method of replacing the epoxy with graphene sheets will undoubtedly result in significant power savings for the GO membrane discovery. Let’s hope that this innovative technology soon enters our homes as well as the market.
Metal Organic Frameworks
We use cold metal organic frameworks, also known as MOFs. These materials are constructed from two distinct parts, even though metals are mentioned in the name. And that is one component. The organic linkers are the second component. And this is where we use it to our advantage; it’s almost like constructing with Legos; you have different pieces that go together simply, but in the end, you can create incredibly intricate constructions.
Instead of working with Legos into centimetres, consider moving down to the Nano regime. Therefore, even though this is considerably smaller, we still deal with self-assembly. This implies that everything in the reaction mixture will discover each other because we designed them to do so in a particular way. Finally, you receive one product, or nearly 100% yield.
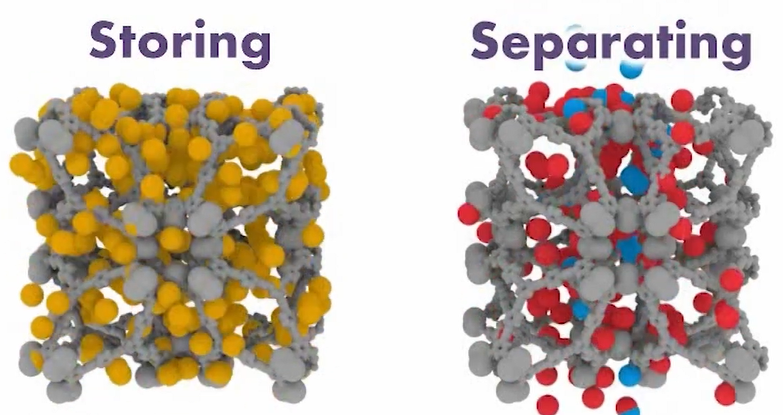 Therefore, using very basic building pieces, this is the simplest approach to develop incredibly complicated systems. From there, you might consider your alternatives and the vast array of possibilities, even if you just have a tiny collection of building pieces. However, we don’t rely just on throwing things out there to see what sticks. We set them up such that they would interact in a particular way.
Therefore, using very basic building pieces, this is the simplest approach to develop incredibly complicated systems. From there, you might consider your alternatives and the vast array of possibilities, even if you just have a tiny collection of building pieces. However, we don’t rely just on throwing things out there to see what sticks. We set them up such that they would interact in a particular way.
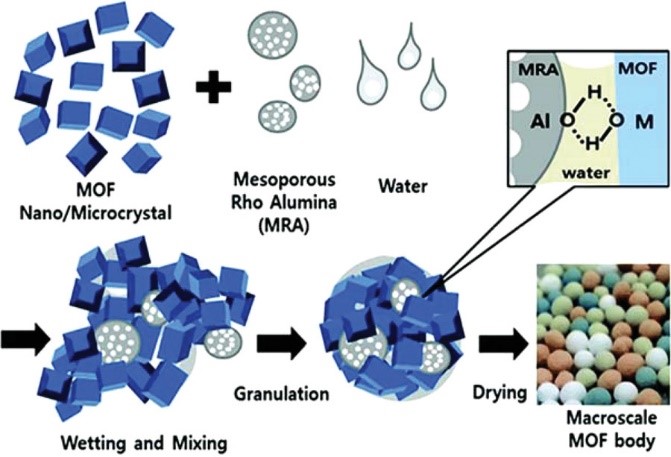
The only issue with a sponge is that it is not uniform, and unlike materials that function with MOFs, it is very difficult to manage the number of cages inside a small unit volume. You have the ability to change the cage’s size, as well as the size of its window and door, allowing you to programmatically turn it on and off as needed.
Micro-ZVI remedial processes
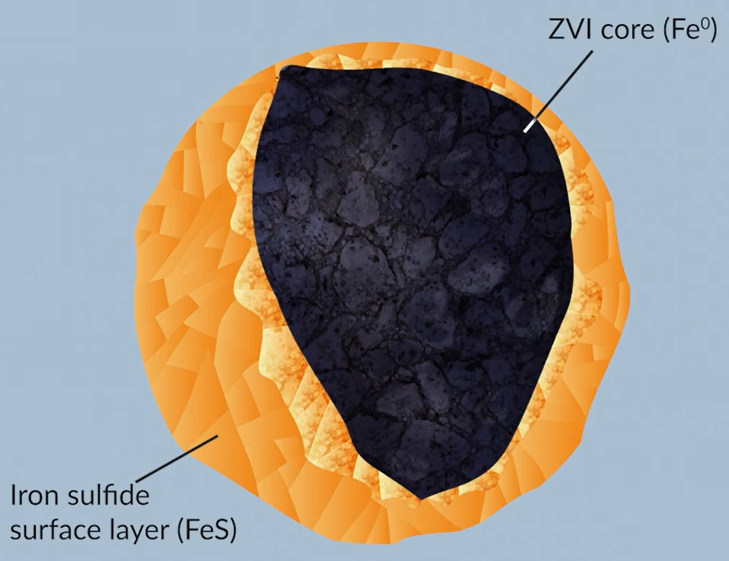
Micro-ZVI remedial is a powerful sulphonated micron ZVI product designed to treat chlorinated contaminants. As micro-ZVI contains particles ions that are less than five microns in diameter, providing excellent reactivity and distribution coupled with powerful chemical reduction.
 Each individual particle is coated with a thin iron sulphide layer, providing several benefits to the treatment process.
Each individual particle is coated with a thin iron sulphide layer, providing several benefits to the treatment process.
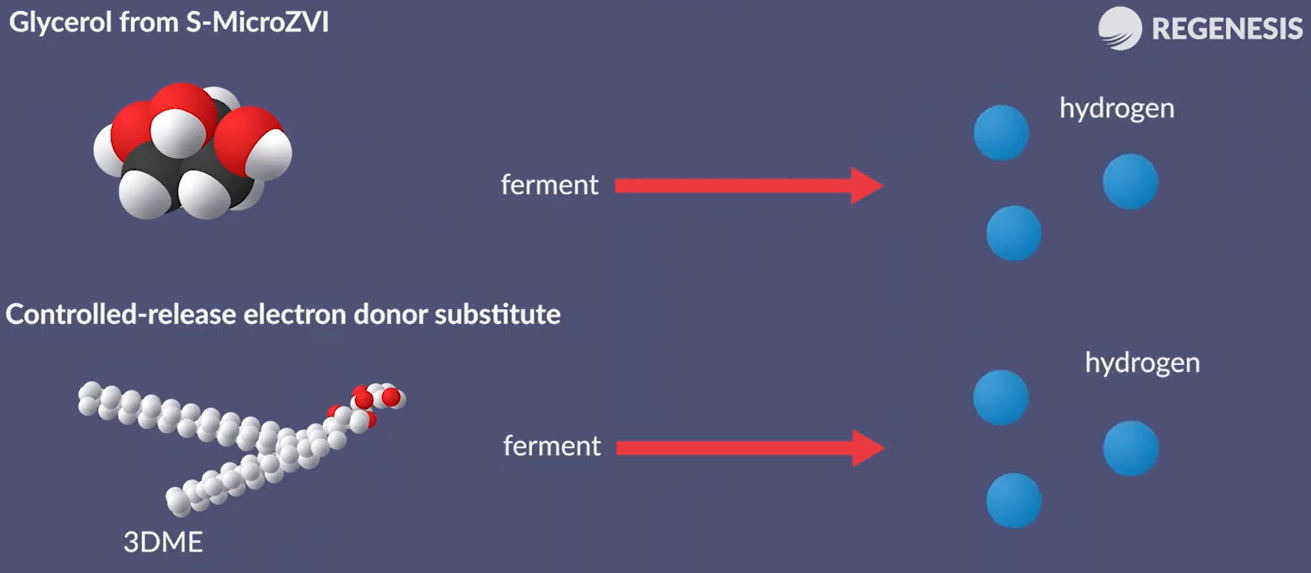
 Sulphation enhances free activity with chlorinated eating’s minimises passivation through reaction with water and enhances persistence of the treatment. The sulphonated Zero valent iron colloids are suspended in a solution of water and glycerol.
Sulphation enhances free activity with chlorinated eating’s minimises passivation through reaction with water and enhances persistence of the treatment. The sulphonated Zero valent iron colloids are suspended in a solution of water and glycerol.
The resulting liquid is safe and easy to apply and provides both a biotic and biological reduction of the contamination. As micro-ZVI is often co applied with 3d Micro emulsion an effective and long-lasting controlled release electron donor. It is prepared by first mixing the electron donor micro emulsion then simply adding in the s micro-ZVI.
Electron efficiency describes the relative amount of electrons provided by the zero-valence iron core that actually reduced the target contaminant compared to those that are lost to reaction with water through hydrolysis Bare.
 ZVI has a low electron efficiency with as much as 99% of the electrons provided being used to degrade water. Reduction of the target contaminant is minimal and so most of the potential chemical reduction is lost.
ZVI has a low electron efficiency with as much as 99% of the electrons provided being used to degrade water. Reduction of the target contaminant is minimal and so most of the potential chemical reduction is lost.
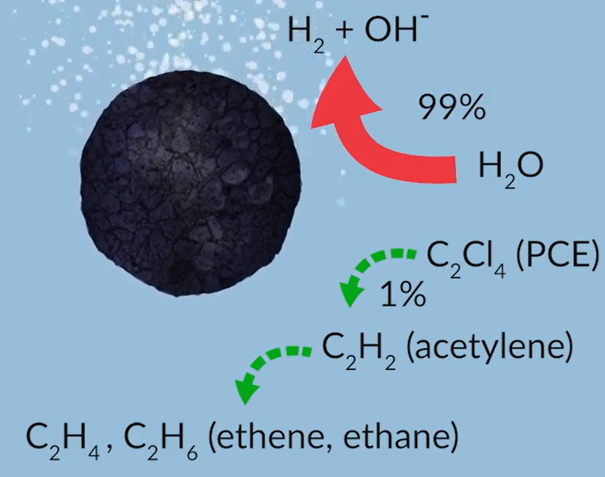 With s micros ZVI the situation is reversed due to the hydrophobic iron sulphide layer polar contaminants are absorbed and electrochemically reduced while hydrolysis is minimised This results in a higher electron efficiency and enhanced reaction rate with a chlorinated ethene.
With s micros ZVI the situation is reversed due to the hydrophobic iron sulphide layer polar contaminants are absorbed and electrochemically reduced while hydrolysis is minimised This results in a higher electron efficiency and enhanced reaction rate with a chlorinated ethene.
Once injected into the subsurface the glycerol in the s micro-ZVI and the 3d micro emulsion ferments to produce molecular hydrogen. The hydrogen is used by the halogenated bacteria to sequentially degrade the contamination by enhanced reductive dichlorination.
 A biotic electrochemical reduction of the parent compounds is provided by contact with the particles of s micro-ZVI. This abiotic degradation pathway does not produce the more toxic daughter products of the enhanced reductive dichlorination.
A biotic electrochemical reduction of the parent compounds is provided by contact with the particles of s micro-ZVI. This abiotic degradation pathway does not produce the more toxic daughter products of the enhanced reductive dichlorination.
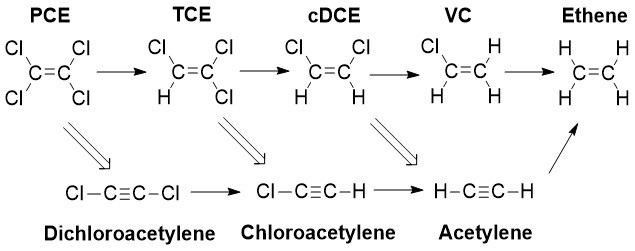 This process can also be known as the better pathway. S micro-ZVI provides both biological degradation of chlorinated contaminants and chemical reduction which occurs simultaneously. This combination of degradation pathways reduces the production of daughter products provides a more rapid reduction in contaminant mass and allows higher levels of contamination to be targeted.
This process can also be known as the better pathway. S micro-ZVI provides both biological degradation of chlorinated contaminants and chemical reduction which occurs simultaneously. This combination of degradation pathways reduces the production of daughter products provides a more rapid reduction in contaminant mass and allows higher levels of contamination to be targeted.
ZnO Nanoparticles
So, that is about to similarly you can see ZnO is utilized in this research work and that is H2O Basically, it will generate the hydroxyl radicals that is OH. through these excitations that is the valence band covalent band etc. light is there.
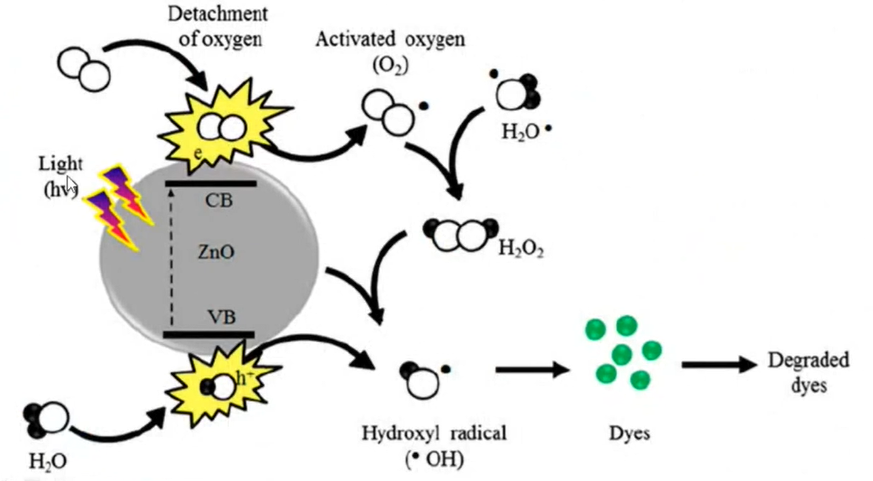 So, it will generate the electrons and whatever oxygen is present, it will form H2O2 also in the solution from that H2O2 then OH radicals are generated and when these OH radicals come in contact with the dye it will degrade the particular dye. So, all these processes are the oxidation processes because the oxygen is getting involved when the hydrogen is getting involved it is a reduction process.
So, it will generate the electrons and whatever oxygen is present, it will form H2O2 also in the solution from that H2O2 then OH radicals are generated and when these OH radicals come in contact with the dye it will degrade the particular dye. So, all these processes are the oxidation processes because the oxygen is getting involved when the hydrogen is getting involved it is a reduction process.
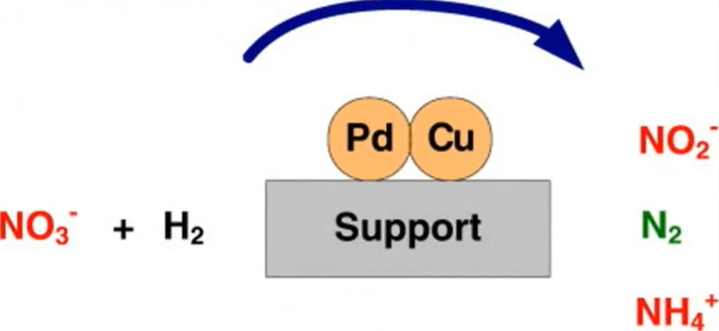
Bimetallic nanoparticles
we have discussed zero valent metal nanoparticles and these metal oxides now the Bimetallic nanoparticles and their composite. Bimetallic systems essentially use two metals: one in zero valent form, such as Mg+2 or Fe+2, to generate nascent hydrogen when it comes into contact with water through anodic corrosion, and another with a high reduction potential, such as regular metals like AgPd, to act as a hydrogenating catalyst.
So, here the iron copper by metallic system So, CuFe system is there and embedded in this catalyst a composite material is utilized for the degradation of the organic compound. So, this is the material on which CuFe by metallic particles are embedded and if the organic compound comes in contact with this, it will be reduced mineralized to CO2 and H2O and the H2O2 is come in contact it will generate the hydroxyl radicals and these particular CuFe system can be utilized for you can see the various the materials they have tried on phenol, bisphenol, 2-4-6 Tri-chloro phenol, methylene blue methylene orange etc.
 How this biometric system works. So, that is shown in this picture, and it is utilized in the De-chlorination of the chloro phenols here you can see Mg and it is a silver system on the periphery of Mg silver is provided that is Ag deposited. So, AgMg system in this deep corroding Mg is there.
How this biometric system works. So, that is shown in this picture, and it is utilized in the De-chlorination of the chloro phenols here you can see Mg and it is a silver system on the periphery of Mg silver is provided that is Ag deposited. So, AgMg system in this deep corroding Mg is there.
 So, whatever the base metal is there either Fe or Mg it will be corroded and then the particles are generated. So, the whenever this corrosion of the metal is their Fe or Mg it generates the nascent hydrogen, and Silver or palladium, two noble metals that absorb hydrogen, are intercalated by the catalyst to create a metal hydride (MH), which combines with the target substrate to decrease it.
So, whatever the base metal is there either Fe or Mg it will be corroded and then the particles are generated. So, the whenever this corrosion of the metal is their Fe or Mg it generates the nascent hydrogen, and Silver or palladium, two noble metals that absorb hydrogen, are intercalated by the catalyst to create a metal hydride (MH), which combines with the target substrate to decrease it.
So, reduction can also be carried out by corroding the zero valent metal with direct electron transfer. So, in the picture you can see the nascent Hydrogen intercalated within Ag which will form Ag, HX and these HX comes in contact with the pentachlorophenol, and it will be reduced to phenol and free chlorine.
This pentachlorophenol When it comes in contact with this corroding metal also it is also gotten reduced. So, basically in any Bimetallic system, the three main steps are there, the corrosion of the iron leads to the generation of H2, then if the whatever the metals are there, Pd and H2 are combined to form the transitional compound that is PdH2, and it will form the Palladium hydride embedded in Pd crystal lattice and these palladium hydride chlorinated compounds.
So, in this way it is utilized.
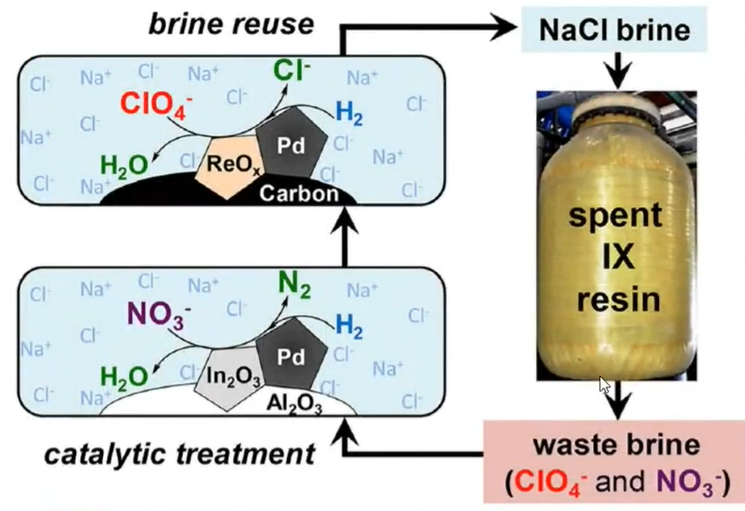 Application of Bimetallic
Application of Bimetallic
The use of a bimetallic catalyst to remove perchlorate from waste regenerating brine from an ion exchange process. So, in the picture you can see the NaCl brine is utilized in spent resins and from the resins it generates the waste brine which contains the ClO4– and NO3–. So, here you can see the Pd and Al2O3 is prepared.
So, it will give nitrate is converted into N2 and the ClO4– is converted into Cl– ion and the NaCl brine is utilized. So, this is the various used to have the nano particles lead that is the Bi-metallic system.
So, all those are having a very good positive aspect, but the limitation is there TiO2 that is high operation cost tough to recovery and sludge production. Similarly, Fe also it is widely utilized, but it is very difficult to recover then by metallic near system also it will generate the sludge because the corrosion of the system is taking place Fe and Fe2+ so, Fe(OH)2 and so, much of sludge is generated then nanofiltration and the Nano membranes, so, membrane fouling is there etc.
 So, there are certain disadvantages of all these materials which we are utilizing. So, these are the methods which we are utilizing So, we must go for the new method in which we can easily recover all the nanomaterials.
So, there are certain disadvantages of all these materials which we are utilizing. So, these are the methods which we are utilizing So, we must go for the new method in which we can easily recover all the nanomaterials.
Water Purification Frequently Asked Questions
1) How Nanoparticles are used in water purification?
The highly porous nanoparticles, which are made to grab water and hold onto it like a sponge, also reject dissolved salts and other contaminants. Additionally, the organic substances and bacteria that eventually clog up conventional membranes are repelled by the hydrophilic nanoparticles incorporated in the membrane.
2) How contaminated water can be purified by nanomaterials?
Nanotechnology-based water filtration makes use of microscopic components like carbon nanotubes and alumina fibres. Additionally, it makes use of nanoporous zeolite filtration membranes, nanocatalysts, and magnetic nanoparticles.
3) Which nanoparticles are used for purification of water?
Zero-valent metal nanoparticles, metal oxide nanoparticles, carbon nanotubes (CNTs), and nanocomposites are now the most thoroughly investigated nanomaterials for the treatment of water and wastewater.
4) Are nanomaterials soluble in water?
Depending on the charges of the citric acid on their surface, the NPs are soluble in water. At normal temperature, the NPs exhibit superparamagnetic characteristics. The dispersity and magnetic characteristics brought on by surface and finite-size effects point to a bright future for NPs in real-world uses.
5) How nanotechnology plays an important role in water purification?
Currently, nanotechnology is essential to water filtration methods. Nanoscale atom manipulation is the practise of nanotechnology. Nano membranes are used in nanotechnology to soften water and remove impurities like chemical, biological, and physical pollutants.
6) How do you separate nanoparticles from water?
For nanoparticle separation, physical gaps or barriers, such as membranes or columns to filter particles based on their characteristics, are frequently utilised in addition to external fields. Chromatography and nanofiltration are two sieving processes that are frequently used to separate nanoparticles.
7) Why nano particles are so important?
Numerous varieties of nanoparticles have made significant contributions to society. In the medical industry, biological nanoparticles are utilised as biosensors and medication delivery systems. Consider the biological markers called nanoprobes and nanospheres, which are comprised of gold nanoparticles.
8) Can nanoparticles occur naturally?
Regardless of human activity, naturally occurring nanoparticles (NNPs) are frequently found in all spheres of the Earth (i.e., in the atmosphere, hydrosphere, lithosphere, and even in the biosphere).
9) Can we filter nanoparticles?
Nanoparticles and practically all sizes of solid particles can be captured by a straightforward HEPA filter.
10) Why are nanomaterials used in water treatment?
The usage of nanoparticles is favourable because they have novel traits that have been demonstrated to be very efficient and effective for wastewater treatment, such as high surface area, high reactivity, and strong mechanical properties.
11) What is the most important property of nanomaterials?
Friction is the key characteristic of nano metals. One of the distinctive characteristics of nanomaterials is their small size. Up to ten thousand times smaller than the width of a human hair, nanomaterials can be. Nanoparticles have a very high surface area to volume ratio.