Ion-Exchange Treatment of Radioactive Wastewater
Overview:
Nuclear technology demands a number of effluent stream treatment processes, such as removal of radioactive pollutants and water chemistry balancing, before it could be discharged back to the ecosystem. Notable contributors in radioactive pollution are nuclear fuel re-processing plants, nuclear power plants, and nuclear technology research centres. The water treatment methods employed could either be for treating reactor primary coolants, spent fuel pool clean-up, or liquid radioactive waste management systems. Ion exchange is a promising technique in this regard, a well-developed practice that has been used for many years in both the nuclear sector and other industries and is one of the most prevalent treatment procedures for such aqueous streams.
Because of the rising usage of radioisotopes in many industrial sectors, radioactive wastewater has raised severe concerns about human health and the environment. All radionuclides are thought to release high-energy particles or electromagnetic waves, and exposure to such radiation can cause chemical bonds to break and proteins in human cells to ionize. As a result, the treatment of radioactive wastewater is intrinsically tied to human health.
Organic ion exchange resins have traditionally been used in nuclear power plant process water systems to manage system chemistry, reduce corrosion and deterioration of system components, and remove radioactive pollutants. Organic resins are also utilized in a variety of chemical decontamination and cleaning operations, such as reagent regeneration and radionuclide elimination.
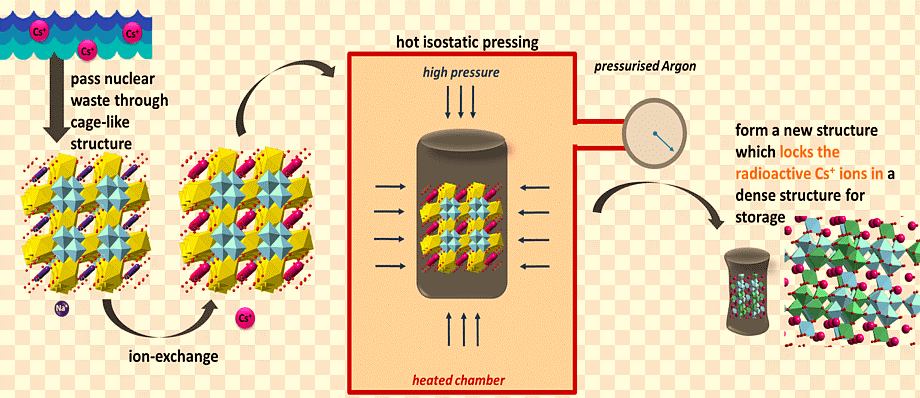
The ion exchange process is extremely efficient at transferring radioactivity from a big volume of liquid to a small volume of solid. The treatment and conditioning of radioactive used ion exchange materials is a time-consuming procedure that necessitates careful evaluation of the materials’ qualities as well as their compatibility with various processing, storage, and disposal options. The management of spent ion exchange media must also be considered in the context of an integrated waste management system for all radioactive waste generated; for example, ion exchange waste may make up such a small percentage of the total waste stream that specialized treatment for ion exchange media may not be cost effective.
Classification of Nuclear Industry Effluent:
The radioactive waste is classified in two categories; based on half-life of the radioactive element and the activity level, as per internationally accepted classification system. This is chiefly due to the radioactivity level in the effluent stream effects the choice of management alternatives for shielding requirements. This approach divides radioactive wastes into three categories: exempt (EW), low- and intermediate-level wastes (LILW), that can be further classified into long and short-lived (LILW-SL) wastes, and high-level wastes (HLW). The characteristics of these waste classes are listed in Table 1.
Table 1: Radioactive Wastewater Classification
| Class | Characteristics |
| EW | Activity levels that are at or below clearance values, which are predicated on a public exposure of less than 0.01 mSv per year |
| LILW | Thermal power below roughly 2 kW/m3 and activity levels above clearance levels |
| LILW-SL | Long-lived radioactive concentrations are restricted (long-lived alpha emitting radionuclides are limited to 4,000 Bq/g in individual waste packages and 400 Bq/g on average per waste package). |
| LILW-LL | Concentrations of long-lived radionuclides exceed those allowed for short-lived waste |
| HLW | Thermal power more than 2 kW/m3 and long-lived radioactive quantities exceeding short-term waste limits |
Who are the main contributors of radioactive effluents?
Table 2: Source of Radioactive Effluent
| Source | Radioisotopes | Features | |
| Radiolabeling & Radiopharmaceutical |
|
Chemical composition is predictable in a small volume | |
| Lab Production Radioisotopes | Chemical composition that is predictable in a small volume | – Small levels of high specific activity and high chemical concentrations
– More volumes of low-specific-activity |
|
| Scientific Research | Radioisotopes with short and long lives are variable |
|
|
| Decontamination and Laundry | Probably a wide range | Large volumes containing complexing agents but with low specific activity | |
| Pilot Plants and Industries | Depending on the situation | Volumes may be huge, and chemical composition may be unknown | |
| Medical Treatment | 99Tcm, 85Sr, 131I,
|
– Patients’ pee in large quantities
– The amount of prep and treatment time is minimal. |
|
| Nuclear Research Centers | Long-lived individuals may be intermingled with short-lived individuals | Ion exchange resin regeneration produces batches that are virtually pH neutral |
Radioactive Effluent Treatment via Ion Exchange Technique:
Exchanging mobile ions from an external solution for ions that are electrostatically bound to the functional groups in a solid matrix is known as ion exchange. When the functional groups are negatively charged, cations are exchanged, and when they are positively charged, anions are exchanged. The separation of these species can be achieved by taking advantage of the fact that ion exchange media have a greater affinity for certain ionic species than for others under certain conditions; for example, the hydrogen form of a cation exchanger will release its hydrogen ion into solution and pick up a caesium ion from the solution according to the equation:
![]() where R indicates the insoluble matrix of the ion exchange resin. The caesium salt’s negative counter ion is unaffected by the exchange since every caesium ion withdrawn from solution is replaced by a hydrogen ion, maintaining electroneutrality.
where R indicates the insoluble matrix of the ion exchange resin. The caesium salt’s negative counter ion is unaffected by the exchange since every caesium ion withdrawn from solution is replaced by a hydrogen ion, maintaining electroneutrality.
Factors affecting the capacity of Ion-exchange:
- Temperature
- Ionic size
- Ionic valance
- Solution concentration
- Extent of cross-linking
- Type of functional group
Ion-Exchange Substances:
For the ion exchange treatment of radioactive liquids, a variety of materials are available. These materials come in a variety of shapes and sizes, have a wide range of chemical and physical properties, and can be natural or synthetic.
The applicability of ion exchange materials for different purposes can be classified. When liquids from primary circuits or fuel pools need to be filtered, nuclear grade organic ion exchange resins are typically utilized. The material to be utilized is chosen for its capacity to remove contaminants and unwanted ions while also controlling pH. Nuclear-grade ion exchange resins are comparable to commercial-grade resins but have stricter particle size and composition requirements. Organic resins are frequently utilized for several treatment cycles, eluting absorbed radioisotopes with appropriate solutions and then returning the ion exchanger to its original ionic form before reuse.
Inorganic ion exchange media can be employed in systems where polluted liquid is purified for recirculation purposes or to reduce the level of radioactive concentration in the liquid to allow it to be reclassified. Ion exchange can also be used in the presence of very high quantities of competing ions thanks to very selective inorganic materials. Inorganic ion-exchanger are used only on time virtually.
 Application:
Application:
For many years, ion exchange technology has been used in nuclear fuel cycle operations and other activities involving radioactive liquid treatment. In nuclear power plant, ion exchange materials are used for the following purposes:
- Purification of the primary coolant (water)
- Primary effluent treatment
- Water from fuel storage ponds is treated
- Demineralization of steam generator blow-down
- Treatment of liquid waste and drainage water
- Boric acid recycling purification
- Polishing of condensate (for nuclear power plants with boiling water reactors)
Most of the systems and methods mentioned will operate with a variety of ion exchangers. The most feasible way to select the ion-exchanger is gather the information from media manufacturer via tests on effluent to be treated.
Limitations:
The usability and efficiency of ion exchange materials and procedures are limited by certain properties. Due to leakage or breakthrough, complete removal of a given radionuclide is typically not possible when utilized as a packed bed in a column. As a result, the radionuclide will pass through without being captured. This breakthrough may be relatively low in a well-designed system, but it will nonetheless exist. Leakage could be caused by:
- Colloidal radionuclides or radionuclides attached to finely split particle matter (pseudo-colloidal).
- Non-ionic or other non-exchangeable forms of the radionuclide may exist.
- A fraction of the radionuclide could be non-ionic or non-exchangeable.
- Radioactive solid is difficult to handle.
- Low selectivity, which is influenced by the high salt concentration.
- Adsorption capacity and rate are both low.
- Adsorbent or ion exchange material regeneration and reuse.
Advantages:
- Easy to use
- Efficiency is high
- Clean
- Cost-effective
- Large range of available ion-exchangers
- Simple and convenient
Cost Consideration:
A full economic study should be undertaken before adopting a certain ion exchange treatment procedure. The following are the main cost components to consider:
- Capital expenses
- The ion exchange media’s initial cost
- The operating expenses
- The costs of treating and disposing of an ion exchanger that has been used.
Ion-Exchange Frequently Asked Questions
1) What is ion exchange in wastewater treatment?
One or more unwanted ionic pollutants are eliminated from water using the ion exchange technique by exchanging them with a less disagreeable or non-objectionable ionic material.
2) What are the 4 types of ion exchangers?
Ion exchange resins (functionalized porous or gel polymers), zeolites, montmorillonite, clay, or even soil humus can all act as ion exchangers.
3) What is the basic principle of ion exchange technique?
The process of ion exchange involves transforming the ions in a solution into a solid, which then releases new ions of a different type but the same polarity. This implies that different ions that were once present in the solid take the place of the ions in solutions.
4) Why ion exchange method is used?
Ion exchange is a common method for demineralizing or softening water, but it is also used in other water treatment procedures as De-alkalization, deionization, denitrification, and disinfection to remove other impurities.
5) What are the characteristics of ion exchange?
- Ion Exchange Resin Characteristics
- Total Capacity
- Salt Splitting
- Moisture Level
- Micro porosity
- Particle Size
- Uniformity of Particle Size
6) Which element is used in ion exchange method?
The resin beads draw uranium from the solution through an ion-exchange mechanism. Then, after being transferred to a processing facility, uranium-loaded resins are separated from the resin beads to yield U3O8 and yellowcake.









