Fenton Treatment for Pharmaceutical Wastewater Treatment
Introduction
The pharmaceutical sector creates a wide range of goods. As raw resources, it employs inorganics or organics, the latter which might be manufactured or of animals and plants origin. The majorities of such pollutants are harmful to biological life and are typically classified as chemical oxygen demand (COD) values, high BOD, and a low BOD/COD ratio, which is the primary reason for biological treatment failure. The presence of such chemicals in the water habitats and their potential impacts on living species are causing considerable worry. Past research has suggested that wastewaters were treated using conventional technique procedures. Since these wastewater recovery techniques are less successful, if not ineffectual, against very persistent refractory and hazardous chemicals, minimal progress has been obtained. Another major disadvantage of these methods is that they produce significant amounts of sludge that must be thermally destroyed before being disposed of. Fenton system is among the most effective oxidative strategies for removing recalcitrant or harmful organic contaminants from the water and effluent. The oxidation of Fe2+ to Fe3+ and the production of a significant hydroxyl radical (HO•) are responsible for the remarkable extraction efficiency of this approach. Since mutually Fe2+ and Fe3+ ions are coagulation factors, the Fenton process can perform both coagulation and oxidation functions in the treatment method.
Treatment Process
Currently, two methods have been suggested to describe the Fenton reaction’s destruction of organic materials. One would be the Harber-Weiss mechanism, which assumes that active oxide species OH are produced in the Fenton reaction to breakdown organics. The high iron oxide transition mechanism, postulated by Bray and Gorin, is another. They proposed that the Fenton reaction generated the strong oxidising iron compounds (FeO2+, FeO3+) instead of OH. With the advancement of spectroscopy or chemical probe methods, it is now widely understood that the Fenton oxidation is initiated by the production of OH. The Fenton mechanism as it has been recognized is shown by

As per the equation given, ferrous iron (Fe2+) is swiftly oxidised to ferric ions (Fe3+), whereas Fe2+ is slowly produced via the Fenton-like process involving Fe3+ and H2O2. The Fenton reaction is commonly thought to be centered on Equation (1). The Fenton method is basically carried out at a pH of 3 in the solution. The oxidation rate of OH is proportional to the pH of the solution. With decreasing pH, the oxidation capacity of OH increases, and the oxidation rate increases. Furthermore, the activity of the Fenton reagent decreases when pH raises leading to a shortage of active Fe2+, resulting in the generation of inert iron oxohydroxides as well as ferric hydroxide. However, at high pH, auto-decomposition of H2O2 occurs. Iron complex molecules [Fe(H2O)6] 2+ and persistent oxonium ion [H3O2]+ occur at very low pH levels, reducing the reactivity of Fe2+ as well as H2O2. As a result, the Fenton process’ ability to destroy organic molecules is diminished at both high and low pH. In addition, the Fenton reaction mechanism has a large number of competitive reactions. The pace of Fenton oxidation is proportional to the iron dose, whereas the amount of mineralization is proportional to the oxidant concentration. Understanding the Fenton reagent’s mutual connections in terms of OH consumption and production is critical. As per the quantity of the [Fe2+]0/[H2O2]0 ratios, these interactions were explored and divided into three categories. Their findings revealed that the [Fe2+]0/[H2O2]0 ratio and organic material had an impact on Fenton reaction route competitiveness.
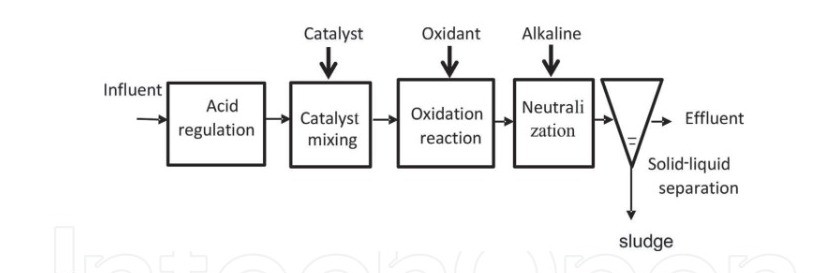
The Fenton’s process schematic process is shown in the diagram.
Applications of Fenton Process
- Improvement in biodegradability.
- Removal of BOD as well as COD
- Removal of colour and odor.
- In radioactively polluted sludge, resin is destroyed.
Advantages of Fenton Process
For the oxidation of organics Fenton has a number of benefits, including
- excellent performance
- convenience; which means it run at room temperature and normal pressure
- non-toxicity
- H2O2 can break down into ecologically nontoxic molecules like O2 and H2O
Disadvantages of Fenton Process
The massive cost of H2O2 as well as the large volume of ferric sludge generated in the neutralization phase of the processed solution prior disposal is the main drawbacks of using the Fenton procedure. These flaws, along with increasingly rigorous water regulations, make developing alternatives to enhance Fenton technology difficult. On the one hand, energy was added into Fenton to boost OH production, like photo-Fenton as well as electroFenton. Iron-based catalysts and the recycling of Fenton sludge, on the other hand, were established as a Fenton-like process.
Fenton Treatment Frequently Asked Questions
1) What is Fenton process in wastewater treatment?
An innovative treatment technology for the treatment of wastewater and water is the electro-Fenton method. Utilizing hydroxyl radicals to oxidise dangerous contaminants, Electro-Fenton is particularly effective at treating chemicals that are difficult to break down in conventional water and wastewater treatment facilities.
2) How does the Fenton reaction work?
In the Fenton reaction, the ferrous and/or ferric cations break down hydrogen peroxide catalytically to produce potent oxidising agents that can break down a variety of organic and inorganic compounds.
3) What is the Fenton test?
The decomposition of hydrated Nafion membrane reveals the mechanism of the Fenton reaction, which is the most popular degradation test for organic materials employing hydrogen peroxide (H 2O 2) and iron (Fe) cations. It has been assumed that this reaction process produces OH radicals.
4) Is Fenton reaction reversible?
A non-radical mechanism for the Fenton reaction was put out by Kremer [29]. He proposed that the process begins with the exchange of an H2O molecule in the hydration shell of Fe2+ ions by H2O2, resulting in the reversible synthesis of the main intermediate “Fe2+ H2O2” from Fe2+ and H2O2.
5) Where does the Fenton reaction occur?
The Fenton reaction, which involves the interaction of Fe(II) with H2O2, occurs naturally in aquatic habitats, biological systems, and water treatment applications (White et al., 2003; Backa et al., 1993; Winterbourn, 1995; Jomova et al., 2012; Lee et al., 2012). (von Sonntag, 2008).