Photocatalytic Treatment in Pharmaceutical Waste Water Treatment
Overview
Every year, around 300-400 million tons of unprocessed organic contaminants are created, causing water contamination issues, particularly near industrial regions. To address this problem, the lot of nations enacted rigorous environmental pollution prevention rules. Furthermore, it draws scientists’ interest to actively investigate the greatest technology in this research field in the hopes of reducing contamination and improving environmental well-being.
Photocatalysts are one of the most appealing ways to breakdown organic pollutants. This is due to its potential, fast, and effective contaminant degrading capability, which is accomplished by enabling both spontaneous and non-spontaneous responses to optimize the entire process. The procedure for wastewater recovery is defined as a sequence of advanced oxidation that ameliorates its shortcomings, such as significant expense, partial mineralization, and a high hydroxyl radical need. The photocatalysis process necessitates the use of light energy to engage the photocatalytic activity; therefore, this is intriguing since the reaction may be regulated with light or photon supplies.
Mechanism of photocatalysis:
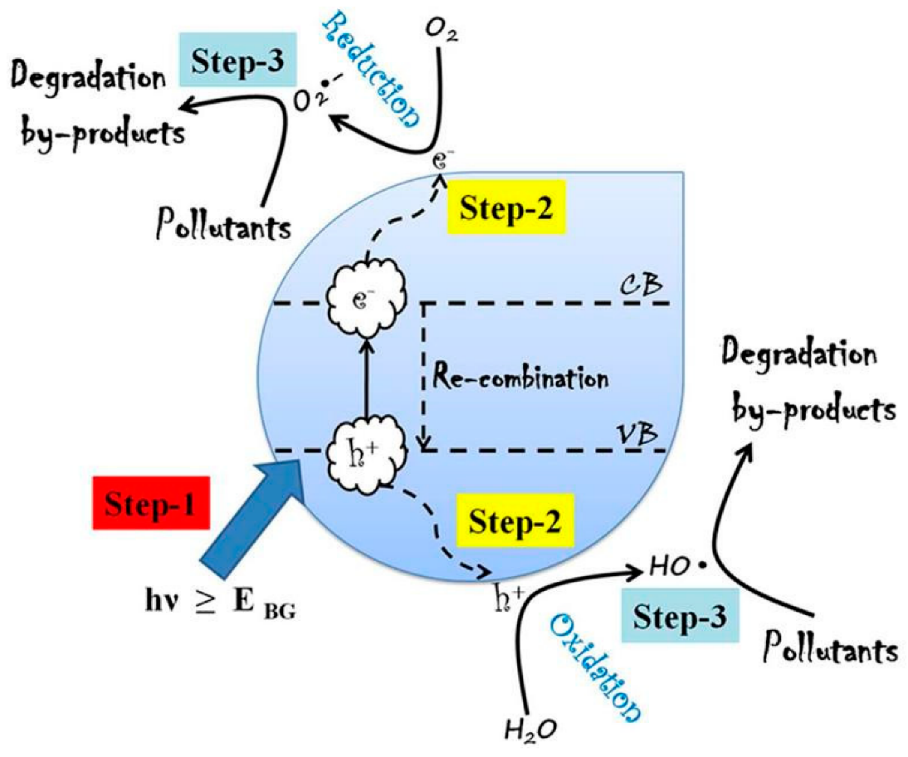
If light strikes the photocatalyst’s surface at a wavelength equal to or greater than the material’s bandgap, electrons in the valence band are stimulated and jump to the conduction band, forming an electron–hole pair. The ensuing redox processes that breakdown contaminants are caused by the produced electron–hole pair.
It’s possible that the technique by which they degrade Emerging Pharmaceutical Contaminants (EPCs) is complicated. In a nutshell, the electron is responsible for reducing dissolved oxygen to make the superoxide anion (O2), whereas the hole is responsible for oxidizing water to produce hydrogen gas and the hydroxyl radical (OH–). The superoxide anion and the hydroxyl radical are oxidative agents that can degrade a wide range of substances. Depending on the contaminant structure, contaminant concentration, water chemistry, experimental settings, and nanomaterial loading, photodegradation can take a variety of paths.
The superoxide anion and the hydroxyl radical are oxidative agents that can degrade a wide range of substances. Depending on the contaminant structure, contaminant concentration, water chemistry, experimental settings, and nanomaterial loading, photodegradation can take a variety of paths. For example:
Sulpha Drug Treatment:
The hydroxyl radicals and holes produced during photocatalysis dominate the breakdown of sulpha drugs (e.g. sulfachlopyridaxine, sulfisoxazole, sulfapyridine). Holes are hypothesized to start the reaction by disrupting the medication’s sulphur-nitrogen link, followed by the introduction of hydroxyl radicals into the structure of a sulfa medication, which eventually dominates the medicine’s breakdown.
Paracetamol:
The hydroxyl radical is also the main reactant in the photocatalytic degradation of paracetamol, producing the hydroxylation and disintegration of the aromatic rings. The molecules generated as a result of paracetamol hydroxylation (for example, hydroquinone) are further oxidized, resulting in unstable structures that dissolve in aqueous solutions. Furthermore, it has been discovered that when the superoxide anion concentration is larger than by acting as a Lewis acid, the hydroxyl radical, or superoxide anion, can destroy paracetamol.
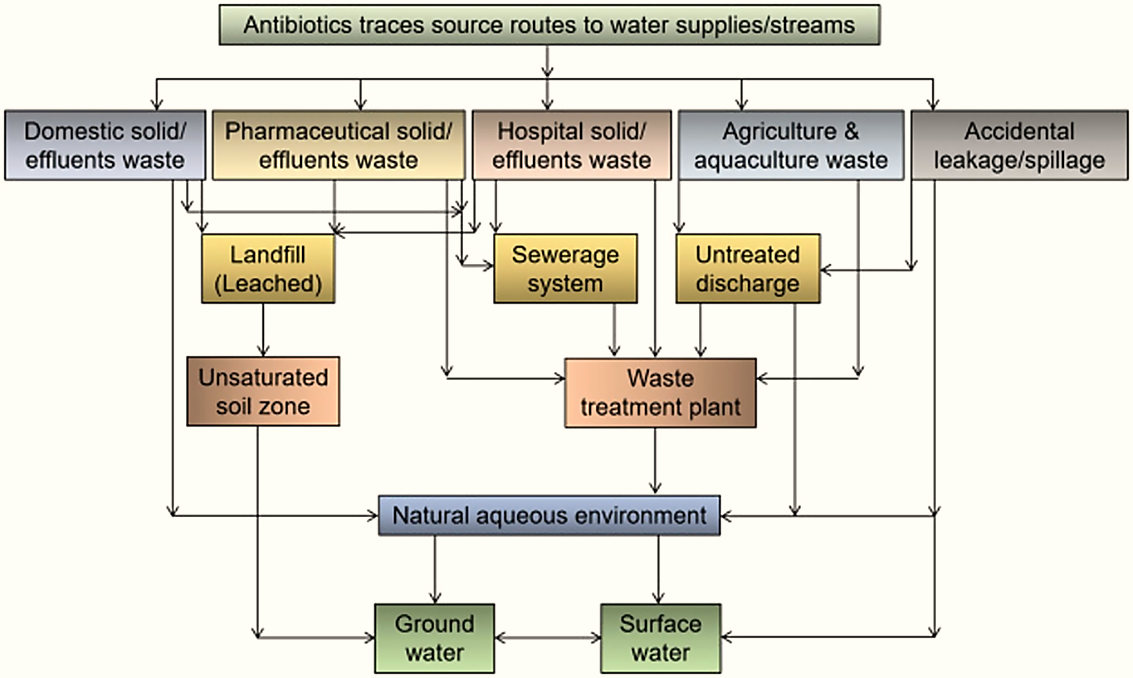
Pharmaceutical pollution in water comes from the following sources:
The global yearly consumption of pharmaceutical pollution has reportedly increased in developed nations such as Brazil, Russia, India, China, and South Africa over the last decade, owing to medical use for prophylaxis/therapy and economic use for commercial aquaculture and cattle farm viability. As a result, PP chemical residues are continuously discharged into wastewater as parent compounds, derivative conjugates, or metabolites via wash-off, urine, and feces.
PP can also enter surface water by direct discharge from factories, hospitals, and domestic wastewater, as well as surface runoff (as a result of the use of polluted biosolids as manure spread on agricultural land), reaching water bodies and groundwater via leaching or bank filtration.
PP can also reach the aquatic environment through the disposal of unused pharmaceuticals in landfills, irrigation with wastewater, off-label emissions, and the disposal of treated animal carcasses. Because of high affinity binding/sorption capacity, aquatic sediments can also store a significant amount of PP. Apart from the application of contaminated biosolids to farming soils, some PP reach the soil and various terrestrial domains by the deposition (air/wet) of short and long-range atmospherically transferred aerodynamically sized PP particles.

Available photocatalysts for the removal of pharmaceutical pollution in water:
- Zinc Oxide (ZnO)
- Strontium Trioxide (SrTiO3)
- Ferrous Oxide (Fe2O3)
- Tin Dioxide (SnO2)
- Copper Dioxide (CuO2)
- Tungsten Dioxide (WO2)
- Ferric Oxide (Fe3O4)
Titanium Dioxide (TiO2) – Commercially available photocatalyst
Titanium dioxide is a versatile compound that has been used in a variety of science, industry, and medical fields, especially stomatology and implant technology. Nano-TiO2 is a substance that can be utilized to substitute biological tissues, such as bones. It can be utilized to restore face tissues and dental implants due to its biocompatibility; it also promotes faster bone formation, enhances implant longevity, speeds up recovery, and has anti-infective properties. Apart from this TiO2 have applications in removal of pharmaceutical contaminations from wastewater.
TiO2 was initially examined for application in solar energy making. The research afterwards shifted to ambient photocatalysis. The use of titanium dioxide as well as its different variations in treating wastewater has been proposed. As a consequence, the majority of research has focused on making TiO2 an acceptable catalyst for photocatalytic degradation of available water pollutants, including developing toxins. The majority of these chemicals are persistent APIs that really are difficult to eliminate from aqueous medium. There are also several substitutes to TiO2 in this purpose, including metal oxide nanoparticles as well as other elements like quantum dots that have been employed in the photocatalytic degradation of pharmaceutical water pollutants.
In the case of pharmaceutical contaminants in wastewater, TiO2 has been utilized to catalyze photocatalytic degradation of analgesics, antibiotics, anticonvulsants, psychiatric pharmaceuticals, lipid regulators, -blockers, non-steroid anti-inflammatory medications (NSAIDs), and many other kinds of drugs.
For Example: (Synthesis Procedure of TiO2)
TiO2 has been employed for the breakdown of ibuprofen (IBP) in water, as well as an Eco-toxicological assessment of the solution. It was employed both in spring water and chemically polluted ultra-pure freshwater. The treatment was conducted using a 125 W medium-pressure Hg vapour lamp to provide synthetic and natural UV light, with a cumulative radiation of 110.67 J cm2. After 1 hour of treatment with synthetic UV light, the quantity of IBP recovered from such an ultra-pure water solution that contains 1000 mg dm3 TiO2 & 1.0 mg dm3 IBP was 92 percent, with an organic carbon deduction rate of 78 percent. The TiO2 concentrations utilized in the treatment ranged from 20 to 1000 mg dm3, with the 1000 mg dm3 concentration proving to be the best. A direct photocatalytic experiment of IBP under sun irradiation was one of the several control experiments carried out.
Immobilized TiO2 was used in the photocatalytic process of three medications in a liquid solution, including chlorpromazine (CPR), atenolol (ATL), and metronidazole (MET) in several investigations. The researchers used pure anatase immobilized on ceramic plates using the sol-gel approach. The TOC extraction rates were 70% and 90% after 8 and 16 hours, respectively.
For the breakdown of several medicines like atorvastatin (ATR), ibuprofen (IBP), diclofenac (DIC), tioconazole (TCZ), ketoconazole (KET), valsartan (VAL), and gentamicin, a functionalized photocatalyst having low TiO2 concentration was created. The effectiveness of photocatalytic degradation in non-imprinted setups using a photocatalytic activity and a commercialized Degussa P25 TiO2 was evaluated. One of the limitations of TiO2 is indeed the limited specificity of photocatalytic processes to target pollutants, which was addressed using molecular imprinting. The molecularly imprinted technologies were created utilizing an acid-catalyzed sol-gel technique with 150 mg of pharmaceuticals, 100 mg of TiO2, and 45 mmol of tetraethoxysilane in a 1:2 volume ratio. Titania was introduced 2.5 hours after the response began. The medications were first put to the test to see if they could photolysis. During irradiation with no need for a photocatalyst, the mean rate of degradation for all investigated compounds was 5.6 percent, which has been regarded minimal. 33 mg of photocatalyst plus 50 cm3 of pharmaceutical were introduced in a bath reactor using airflow (6.5 cm3 s1). The rate of photocatalytic degradation was measured at two following points:
- 1 hour of incubation of a drug in the reaction system for preliminary adhesion to the solution
- Degrading stage with UV irradiation
In comparison to non-imprinted formulations and raw P25 TiO2, the photocatalytic efficiency of molecularly imprinted solutions was reported to be improved for all tested medicines. The imprinted systems were shown to have 5–427 percent higher degrading efficiencies than their unimprinted equivalents. The existence of holes that were specific toward the evaluated medications explained the improved performance. The interactions between the medicines and TiO2 were improved thanks to these locations. Furthermore, following seven catalytic cycles of recycling with diclofenac, the apparatus maintained 60% of its early photocatalytic activity.
Several photocatalytic methods have been explored in which TiO2 has been employed in various forms. TiO2 has been changed in several ways to improve its qualities and eliminate some of its flaws. Simple alterations without the need of a dopant, co-catalyst, or photosensitizer, on the other hand, are restricted in their capabilities. Yet TiO2 has showed several benefits to remove pharmaceutical wastes.
Limitations:
- The photocatalytic efficiency of a material can be further limited by its recombination rate, charge carrier transfer rate, and charge carrier travel time.
- Another drawback of photocatalytic materials is their possible environmental impact. Potential transition products are a significant source of concern, especially if they are released into the environment.
- Degradation can result in hazardous compounds such as phenol derivatives, just as it can with diclofenac.
- Aromatic rings are common in pharmaceuticals, and if not destroyed, they can generate phenolic chemicals, which are known to be hazardous.
- Furthermore, they may produce acids (as with paracetamol degradation), which may affect environmental conditions and endanger surrounding creatures.
- When photocatalysts are released into the environment, their instability in water is another cause of worry. The ions that are released during their dissolution in water have the potential to harm the environment.
Possible Solution:
Prior to their usage in water treatment facilities, the degradation mechanisms of photocatalysts as they relate to EPC and photocatalyst stability in water must be studied. Creating composites and utilizing stabilizing agents, whether natural or artificial, can also help to increase photocatalyst stability and efficiency. Furthermore, by strengthening their stability, their negative environmental consequences can be mitigated.
Benefits:
- Despite their drawbacks, photocatalysts have a wide range of commercial applications. Titanium dioxide, a UV-activated photocatalyst, has been used in commercially available water filtration products and might be used in Advanced Oxidation Process (AOP) to assist destroy a range of pollutants.
- Photocatalysts are becoming more cost effective, and their usage on a broad scale is becoming more realistic, thanks to developments in photocatalytic materials.
- Traditional photocatalysts (TiO2 and ZnO) have several benefits, including chemical–physical stability, low cost, and environmental friendliness.
Recommendation:
The indirect potable reuse method necessitates a grasp of environmental and health regulations in order to ensure water safety. As a result, extensive risk evaluations and health and safety studies are required when using new technologies in order to reduce the technology’s potential risks. While there is no rule governing EPC maximum permissible quantities in water, legislation governing drinking water procedures is typically highly tight in order to protect human health and the environment. The European Union, for example, has established new rules for medicines, demanding more extensive environmental risk evaluations before each pharmaceutical can be used in a continual effort to protect the safety of drinking water and decrease the impact of EPC.
In addition, pharmaceutical pollutants in the environment may be subjected to more thorough monitoring in order to better assess their risk and environmental impacts. Even so, maximum EPC removal may be required, and the use of nanotechnology in water treatment could be crucial in terms of human health and EPC persistence in environmental systems.
Pharmaceutical Waste Water Treatment Frequently Asked Questions
1) What is solar photo catalytic wastewater treatment?
TiO2 nanoparticles coated on plastic granules were used to create a new solar photo catalytic reactor, or photo reactor. Granules of catalyst are inserted into the cavity of a glass reactor panel. Wastewater is moved around the photo reactor using a pump.
2) How does photo catalytic water purification work?
With the use of photo catalysts and UV rays from the sun, this technology can quickly detoxify dirty water* and produce clean water that may be consumed. When Panasonic presented it in Tokyo at Eco-Products 2014, it received a lot of attention.
3) Which catalyst is used in photo catalysis?
Titanium oxide
4) What are the causes of pharmaceutical pollution?
Therefore, drug contamination mostly refers to water pollution. According to a researcher at the Cary Institute of Ecosystem Studies in Millbrook, New York, “Pharmaceutical contamination is now found in rivers all around the world.” Aging infrastructure, sewage overflows, and agricultural runoff are some of the causes.
5) How does pharmaceutical waste get into water?
Drugs that are flushed down the toilet and human excrement are two ways that pharmaceuticals get up in the water system. Pharmaceuticals pass through water treatment even though you might think wastewater treatment facilities would take care of the problem.