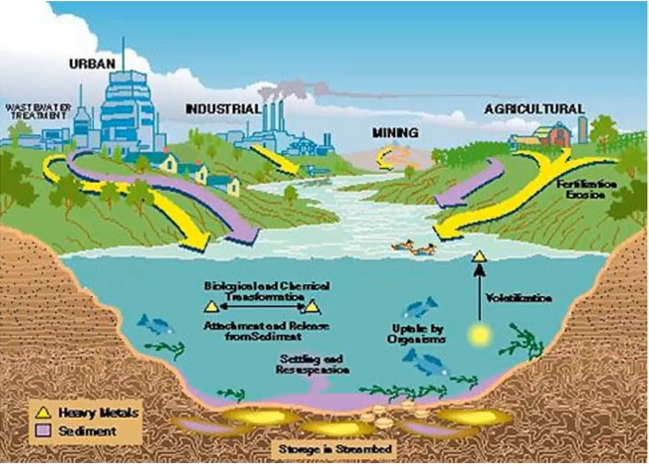
Removal of Heavy Metals from Industrial & Municipal Water
Introduction
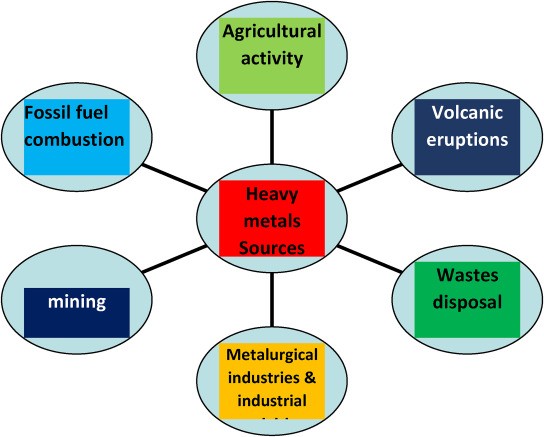
Industrial effluent contains both organic and inorganic pollutants. Among these contaminants are heavy metals, which can be toxic and/or carcinogenic and are dangerous for humans and other living things. Concerning heavy metals from various industries include lead (Pb), zinc (Zn), copper (Cu), arsenic (As), cadmium (Cd), chromium (Cr), nickel (Ni), and mercury (Hg). They are derived from substances including insecticides, fertilizers, mordants, pigments, bleaching agents, fixing agents (which are added to dyes to promote dye adsorption onto the fibres), and fixing agents. Furthermore, metal complex dyes may be the source. In wealthier countries, laws governing wastewater heavy metal limits are becoming more stringent. Lead, mercury, cadmium, nickel, zinc, copper, chromium, cadmium, and arsenic, in that order, have maximum contamination levels in India of 0.05, 0.01, 0.25, 0.20, 0.80, 0.006, 0.00003, and 0.050 (ppm-mg/mL).
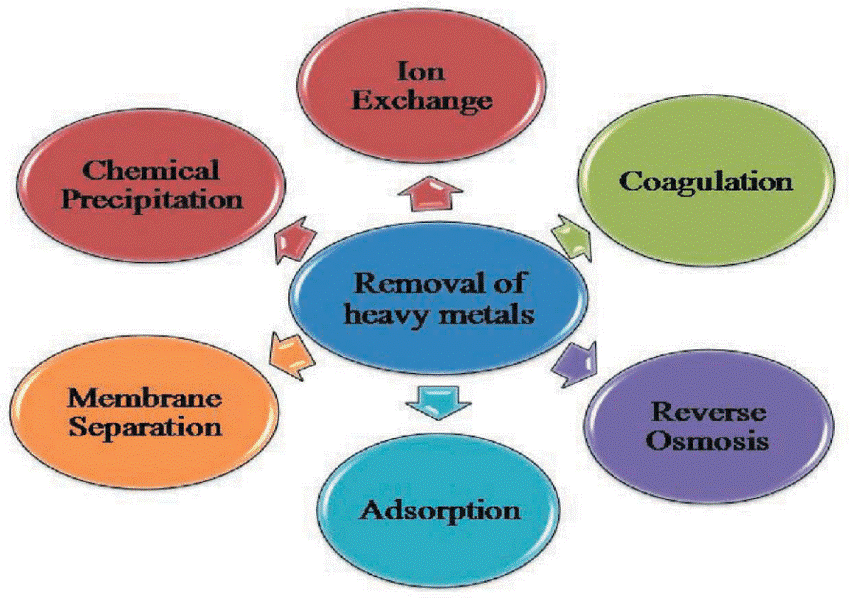
Adsorption is the most effective strategy out of these because the others have built-in drawbacks including producing a lot of sludge, being inefficient, needing delicate working conditions, and being expensive to dispose of. The adsorption technique is developing as a potentially preferred alternative for the removal of heavy metals because it provides design flexibility, high-quality treated effluent, is reversible, and may replenish the adsorbent.
Waterman engineers Australia is the author of this paper on the removal of heavy metals from industrial and municipal water. Background: Drinking water poisoning by heavy metals is a problem for health worldwide. Cadmium, zinc, lead, chromium, nickel, copper, vanadium, platinum, silver, and titanium are among the metals of interest.
Primary sources include industrial discharge, we need an economic method for industrial discharge, and drinking water treatment to take these heavy metals out of the drinking water supply and providing safe drinking water for everyone.
Precipitation Flocculation, Flotation, and Chelation Reactions
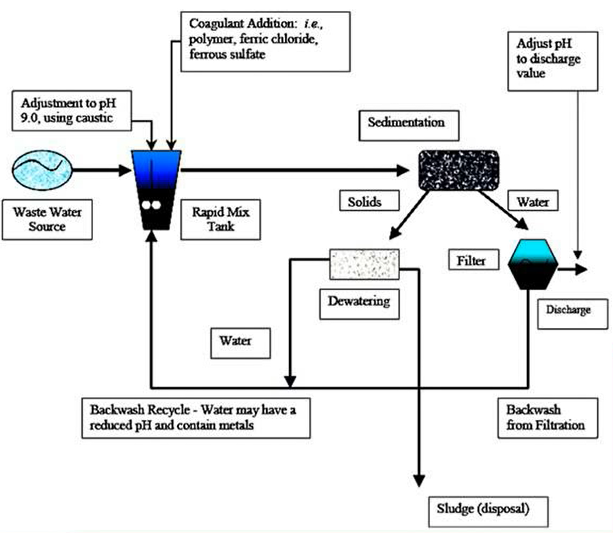
One of the primary methods for removing heavy metals includes a combination of
- Precipitation
- Flocculation
- Flotation, and
- Chelation reactions.
This figure shows the several procedures required in removing heavy metals utilising one of the more often used techniques, which combines precipitation and flocculation with a pH of 9 and a caustic from your water source in order to help the material exit the system.
Ion Exchange Columns
Regenerated ion exchange columns and electrical electrolytic plating on an anode are two other techniques for eliminating heavy metals. But as we’ll see in a minute that the benefits of that are sometimes outweighed by the problems that are into issues with some of the various methods include large quantities of chemicals are needed and toxic sludge from limestone precipitation methods, where we have a caustic bath that’s used to precipitate the heavy metals out of solution.

Corrosion affects electrolytic recovery, particularly at the anode, and the ion exchange columns decrease quickly and are a low volume solution. Low-cost adsorbents made from agricultural waste, industrial by-products, natural materials, or modified biopolymers are a few of the novel strategies that have been investigated.
Process Steps
Some of the process steps that are involved are somehow you need to
- From the bulk solution to the sorbent surface, transport the contaminant.
- Adsorption on the surface of the particle.
- Moving inside the sorbent particle.
There in lies some of the additional options for the adsorbent surface options,
- Zeolites, clay polymer, composites
- Different phosphates like calcined, activated zirconium
- We have chitosan from crab shells, membranes for reverse osmosis and Ultra nano filtration,
- Electro dialysis and
- Photo catalysis which is done over a titanium dioxide type of catalyst.
Adsorbent Surface Options
Now, with the adsorbent surface options, we also can look at crosslinked polyacrylates or hydrogels, otherwise known as Super adsorbents in the diaper industry, such as used by companies such as Kimberly Clark and Procter and Gamble, and they work through water diffusion into the hydrogel carrying the heavy metals.
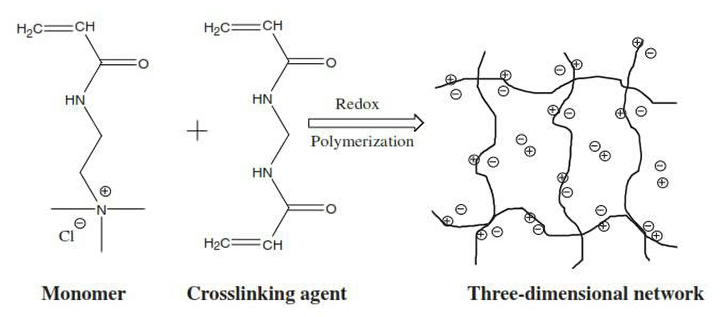
So, you can see where the monomer on the left-hand side with the ionic component is then reacted with a crosslinking agent, which then causes a three-dimensional network and these super adsorbents materials form little round beads as we’ll seen. These round beads can absorb somewhere between 30 to up to as much as 60 grammes of deionized water.
So, there are multiple methods for treating wastewater discharge. According to the literature, lime precipitation is the most practical traditional method for treating organic wastewater containing more than 1000 mg/l of metal.
The hydrogels and the membrane filtration, which are recent adsorbents and are regularly researched and used. Hydrogels can be regenerated with hydrochloric acid, much like the ionic exchange columns. Photocatalysis is another promising innovative technique that is being developed in the last few years.
SAP and CuCl2 Solutions
We’re going to look at the super adsorbent’s (SAP) polymer or hydrogel and copper chloride solution, which shows the removal of copper chloride ions, or heavy metals from the solution. we weigh out 1.5 grammes of super adsorbent and then we pour the water into the super adsorbent in order and that’s 90 mils of water that we’ve put in there then we swirl it around to see what happens in uncontaminated water.
You see the super adsorbent as you swirl, and it starts to gel. Hence the hydrogel name when you see it starting to solidify. This is used like in some of the party tricks where people pour water into a cup and then they turn it upside down. And you can see where the super adsorbent has solidified and adsorbed all the water and it won’t come out of the cup.
Here now we’ve put the superabsorbent into a copper chloride containing solution and you can see the beads have turned blue. We’ve now filtered the water and the one on the left is the one that has copper chloride ions and the one on the right is the one that has been treated with the super absorbent and we should be able to tell that the right one is clearer than the left one.
EFFICACY OF NATURAL COAGULANTS FOR WASTEWATER TREATMENT AND HEAVY METAL REMOVAL
The efficiency of natural coagulants in treating wastewater and removing heavy metals during the coagulation process will be covered in this lesson. To do this, we will study two literary works.
- How well natural coagulation works during the coagulation process.
- Second, utilisation of moringa as a naturally occurring plant for the removal of heavy metals and wastewater treatment.
The purpose of this section of the article is to evaluate how well natural coagulants clean wastewater and remove heavy metals during the coagulation process.
Water is an essential resource for life, and it tends to call the organism however, this resource is becoming limited because of the anthropogenic activities. Therefore, there is a need to develop a low-cost environment and the chemical which can feed the water rapidly and do not change its properties such as physical, chemical, and biological properties.
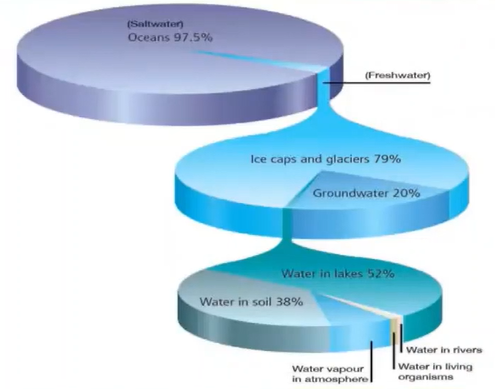
We know that out of 100% of water 97% Is the saltwater and about only 2.5% of the freshwater out of which 79% of the water is confined in the icecap and Glacier whereas only 20% of the water is accessible by humans and it is present in the lakes.
Coagulation
The coagulation is one of the primary steps which is involved in the water treatment or wastewater treatment.
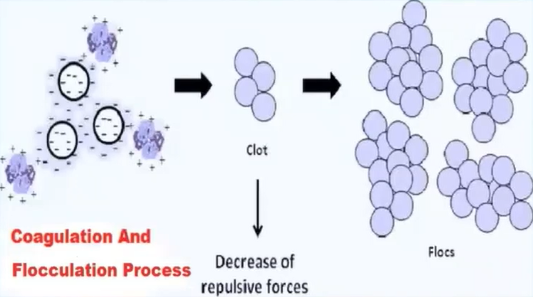
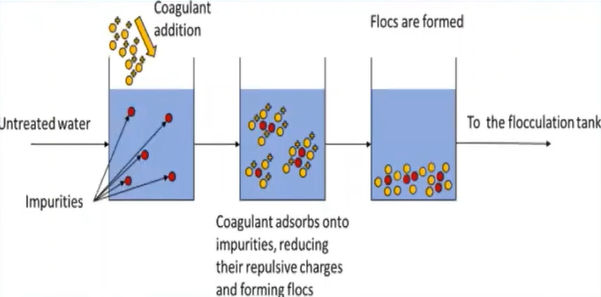
Coagulation is simply defined is a process in which the suspended colloidal particles are destabilised by the addition of chemicals which are called as coagulants, which settle the colloidal particles by neutralising their negative charges.
The primary goals of the coagulation process are to clear the water body of any suspended colloidal particles and to lessen turbidity.
The positively charged covenants are introduced to the water body, which cause the attraction of the negatively charged colour the particles and form the clot and these clots, again combined with other clots and form flocs and they settled down is really at the bottom of the tank, because of the heavier mass.
Coagulants and its types
The coagulants are those substances that are introduced to water to combine non-settling particles into heavier, bigger masses of solid known as flux that are easy to settle.
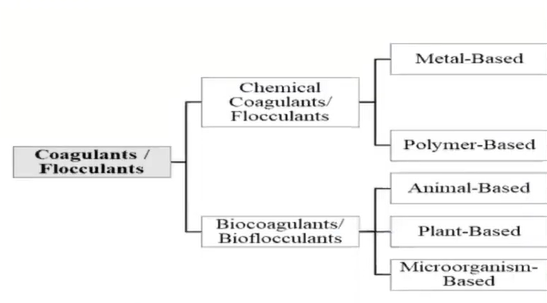
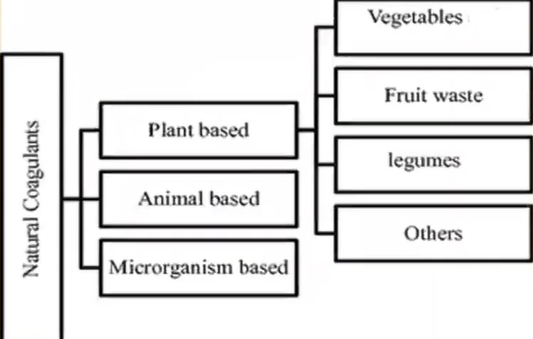 These coagulations are derived from two origins that is from
These coagulations are derived from two origins that is from
- Chemicals and
- Naturally
Chemicals are those which are metal based, and the polymer based whereas the natural coagulants are derived from other animal-based plant based and microorganism.
We are going to learn about only plant based which are either from vegetables, fruit waste, litmus, or others.
Whereas other coagulants such as the orange peel was also effective for the removal of the turbidity by 97%. We have found out that if the pH is maintained between five to seven and dozer is provided between two to three grammes per litre, the moringa oleifera seed could remove 54% of the TSS, total suspended solids from the coffee fermented wastewater.
Similarly, it could remove the turbidity of 99% by using the drafting process, that is, if the moringa oleifera is drafted with the sodium chloride and dosages provide between the 15 milligrams per litre and a pH of 7.5, it could remove large amount of the colloidal particles which are known as the turbidity.
The banana pith was observed that if the pH was maintained of 4 and 0.1 kg per metre cubed, a dosage was provided it could remove 80% of the turbidity sulphates, nitrates lead, zinc iron, chromium, and copper removal.
Similarly, orange peel removed 97% of the availability from the daily wastewater If the pH was maintained between 7.5 and the dosage was provided 0.2 grammes per litre So, now after knowing the efficiency of the plant base coagulants, we should know about the working mechanism of the coagulants.
Mechanism of Coagulation
There are three main types of working mechanisms of the coagulants which are
- Neutralization of Charges
- Cleanse the Coagulation.
- Bridging using polymers.
But the plant based on natural coagulants only costs two working mechanisms which are the polymer bridge and charge Neutralization.
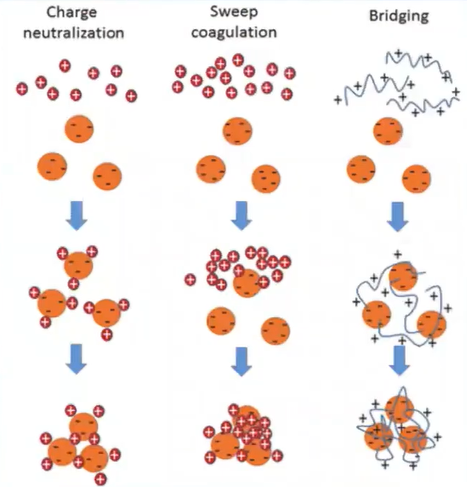
charge Neutralization is simply defined as a phenomenon in which the positively charged as natural covenants are introduced to the water body which causes the attraction of the negatively charged particle and settled down.
Where’s polymers bridging is somebody to conduct a process in which the polymer-based plant national coagulants are introduced a water which caused the attraction of the negatively charged particles and cause they’re settling in large flocs form.
Materials and Method
The moringa oleifera was considered because it had high effectiveness. So Moringa oleifera is our drumstick or went tropical tree which is available throughout the year.
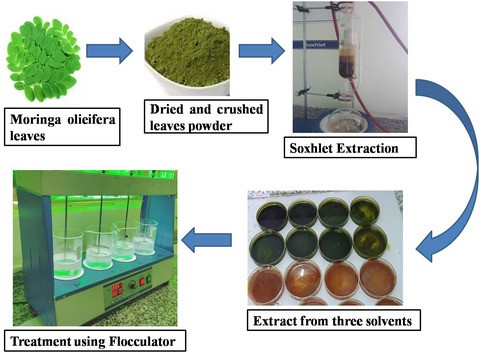
It has various important in the water as well as health importance such as it is in the health it is utilised as a painkiller or antidepressant pill and in the water treatment, it reduces the turbidity TDs and toxic metal from the water. Along with that it has some impacts on the future chemical parameters of the water such as BOD, COD, and pH
So, in order to prepare the coagulants, different phenomena occur first of all, grinded it and shredded particle or we can say our fine particles, then in spite that it contains is removed, in order to remove that the grinded natural coagulants are mixed with the ethanol in a centrifugal compartment. So, that the wild floats above natural coagulants then microfiltered and it is dried and then it could be used for the water application.
First of all, the different concentration like 50, 100, 150 milligrams of MOCK were dissolved in 0.005 litre of distilled water, so, that one person 1.5 And 2 % concentration could be developed prospectively.
To do examination about how the effective are these natural coagulant for the treatment of water and wastewater six jar test was conducted in which six jars were filled with 500 ml of water sample from ear three from the water body and three from the leachate side or we can say which side and in which stock solution that is one 1.5 and two persons were added. They were operated at an initial speed of 150 RPM for two minutes, then they will reduce the speed to 50 rpm and continue for 35 minutes. And then they were left for settling from which the efficiency will determine the parameter analyse what the TDS, EC, Tubridy test, COD heavy metal and BOD.

So the results are the finding of this part of article was that the Moringa oleifera seed cake has no influence on the pH of the single bulk river, but it reduced the BOD to a larger extent and it reduced the COD when added about one person concentration, but when the concentration raise the COD increased, it was because the excessive presence of the chemical which causes the chemical oxygen demand of the microorganisms, whereas the BOD was reduced to from 100 milligrams to 48 milligrams per litre, by the addition of 1% of the concentration when added 2 %, it will cause the reduction by 56.85% and similarly, for the 3% reduced up to third 76.65%.
So, you can see that means, the seed cake has larger impact on the beauty reduction and COD is reduced to a lesser extent there as you can see that the turbidity was reduced to a larger extent that is, it was reduced from 67 to 8.98, NTU or 6.48 and 5.07 respectively according to 1 or 2% and 3 concentrations, but it also had some impact on the conductivity, salinity, and TDs value.
Because, as we add these amounts of the natural coagulants which caused the salinity increase, the acidity of otter has increased, which caused the partner to become acidic, and the total dissolved solids are increasing, and it would increase the BOD. DO was increase because the aeration was carried out means the open jar test was conducted, so, that the amount of oxygen was present. So, DO concentration was increasing.
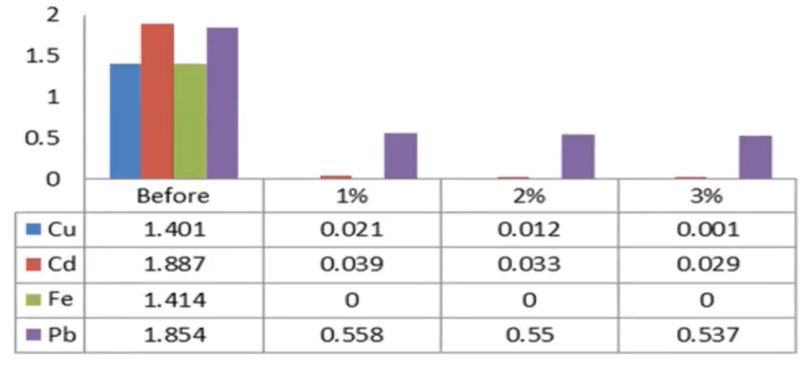
In the heavy metal series, we can see that the copper cadmium and iron lead were reduced to a larger extent, because the full concentration of these parameters verse 1.40 milligrams per litre, 1.88 cents, when milligrams per litre 1.4 and four milligrams per litre 1.854 milligrams per litre, but after the addition of the MO seed cake it was observed that the heavy metals were reduced to a large action the iron was removed completely from the water body, where as the copper cadmium reduced to a larger extent.
Similarly, we can see that, in the landfill site wastewater, the MO seed cake had larger efficiency, it doesn’t affect it, the pH where does it influence the BOD and COD because the BOD is increased, because the amount of the Oxy in these mechanisms are largely present.
So, if we are providing a natural coagulant, so it increases their organic content of water causing the raise of the BOD, whereas the TDS was decrease and the turbidity was decreased is the efficient point of the MO seed cake. And in the heavy metal’s removal, we can see that the copper cadmium and iron and lead were reduced to a larger extent whereas the iron was completely removed.
I would like to conclude that we should conduct detailed study to determine the increasing the organic matter and the chlorine dosage that would be required to remove it. Second, to first of all, promote the plantation of Moringa oleifera seeds or we can plant in the tropical countries so that huge quantity of natural coagulants could be developed.

It was observed from the study data, the turbidity was reduced to 95 or 85 to 94%. And similarly, the pH was not affected, and some physical chemical parameters were influenced, Due to the organic matter content and MO Seed cake, the COD and BOD of the wastewater increased, while the iron was totally removed, copper cadmium was removed up to 98%, and lead was removed up to 78%. 1% of the MO seed cake was sufficient to eliminate the heavy metals.
Heavy Metals Removal from Wastewater
We look at the periodic table, and some of the key stars are listed here. So, even though there isn’t as much Chrome around as there once was, it is still used, particularly for military purposes. There is still a lot of chrome in use. Copper, zinc, and nickel are all widely used materials today. Lead, gold, silver, cadmium, palladium, and selenium. The world’s major producers of nickel and zinc are mostly responsible.
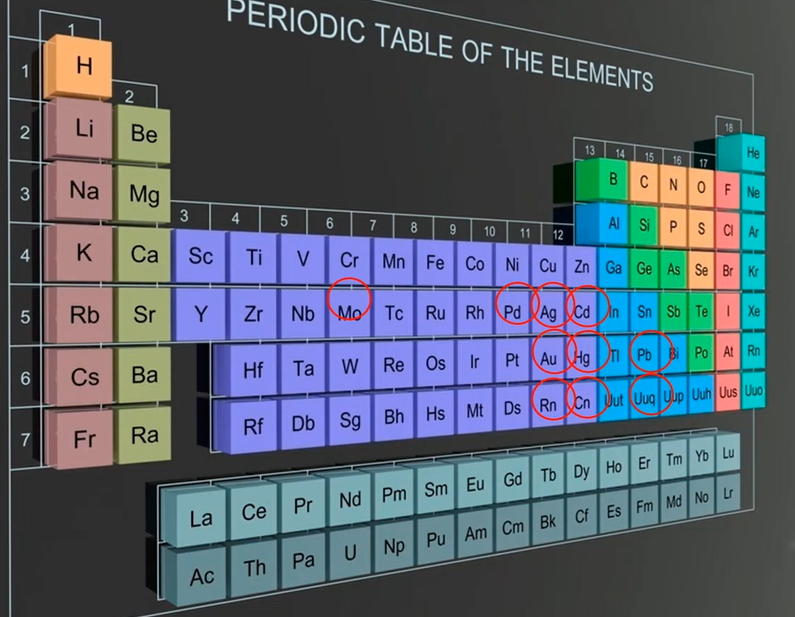
Where Do They Come From?
They come from the power industry, copper piping, because they have copper piping, sometimes they’ve got galvanised piping, sometimes they’re nickels higher, or zinc is higher than coming in and going up, believe it or not.

Why are They of Concern?
You have probably heard the phrase “below things bio accumulate” before. After entering the watersheds, they move into the sediments where they interact with zooplankton known as water fleas and water bears, or daphnia magna. Fish and then birds eat the many microorganisms that make up zooplankton. Additionally, we occasionally consume both fish and birds. And that’s bad for us, particularly if you’re pregnant.
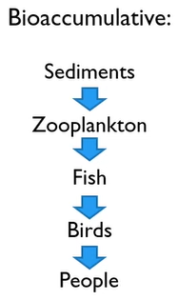
The some of these metals are worse than others cadmium, and Mercury’s and leads are bad. You don’t see those very often and Chrome. But Chrome is easy to treat.
How Do we Remove Heavy Metals?
Precipitation
They must be precipitated in order for us to remove them from the water; otherwise, they will remain soluble and remain in the water. We have a remedy. That contains a lot of zinc, therefore we precipitate out a precipitate after entering precipitant. That metal is being removed from the solution. Here, we’ll explore a few strategies for doing that in a moment.
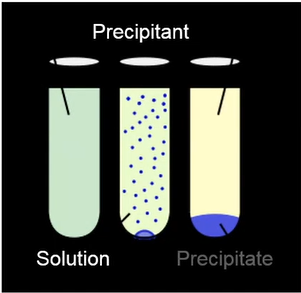
Hydroxide precipitation
If a person has metals that precipitate out at various pH levels, the presence of lead and chrome in the same environment can be problematic.
When we discuss hydroxide precipitation, we are discussing a very intimate lab setting where they are developing these curves.
Chrome serves as an excellent example of how it breaks down. And 0.1 is either the highest or lowest concentration of Chrome. And after that, as you see the pH rise, it returns to solution. In terms of their solubility and kind of U-shaped form, that is what amphoteric metals are.
Advantages of hydroxide precipitation
What are the benefits of hydroxide precipitation? It’s rather affordable. Additionally, most of the time it is simple to treat a tonne of metal. What drawbacks are there? If the sludge is kept in a sludge thickener, the pH may alter if the sludge’s pH changes. These metals might also stabilise. If you pass this waste through your filter press, for instance, you might get a higher reading.

The filtrate may contain more of these metals than you would desire. Sludge is produced in large quantities as well. But once more, if it’s not an FW6 classified hazardous sludge, it might be acceptable since you’re just throwing it in a dumpster. In any case, it is not too pricey. The solubility or insolubility of certain of those metals may be drastically off; for example, one metal may be at pH 6, while another may be at pH 11, which makes the next step challenging.
Sulfide Precipitation
Sulphide is the second kind of metal precipitation. uses the solubility constant once more to remove it from the water by precipitation. In essence, that means that they treat the metal in a supersaturated solution at the maximum possible concentration. So that was essentially the subject of all that jargon. I’ll give you a summary.
Unless you’re extremely lucky and you attract high precipitation works out really well, where you’ve got filtration or other things, you may almost have to go to the sulphide if you have really low metals limits because it’s the only thing that will allow you to get to some of those really low numbers, like.01 or you know, 0.0 anything.
On the other hand, there are two different kinds of sulphide precipitation, including both inorganic and organic forms. However, in general, sulphide might be your best alternative if you’re prepared to reduce those numbers.
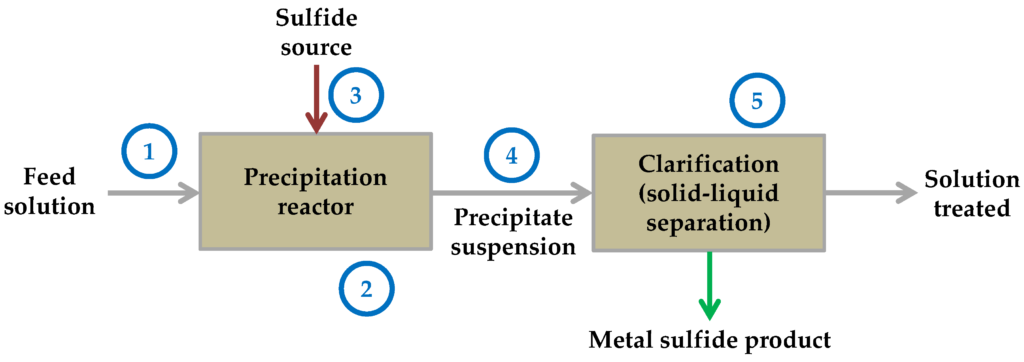
Advantages and Disadvantages
You cannot disregard your pH probe since inorganic sulphide precipitation is less pH dependant than hydroxide precipitation. But you can because it won’t be as important. If you run into sludge from your sludge thickener through your sludge press, you simply don’t produce as much sludge and those metal sulphide complexes, whereas with hydroxide, it might not be exactly what you thought it was. due to the possibility that the metal can dissolve again.
Sulphide therefore forms an insoluble combination with an unknown hydroxide. We face difficulties because hydroxide is a little more expensive. I have customers that, as a result of the order that is, that comes off, have issues with order and need to put equipment in to take care of it. It can be a little bit of a handling issue if it causes things like your fingers to turn black.
One advantage is that, if you do have any crucial lines in the water—and we’ll speak about killers in a second—you’re really, really good at breaking those deadly links. It will pretty much complex with any metal at that pH, less muck, that hydroxide, if you have a somewhat neutral pH. Once more, comparatively pH independent. And you won’t need to worry about that if you wish to put that sludge through a filter press. Those metals returning at a reduced price.
Therefore, these benefits, which are also a little more expensive, and hydroxide I’ve done some studies comparing the two different types of sulphur, organic and inorganic, and they perform nearly equally well. Although I’m not tripping on particularly similar grounds, organic is more expensive than inorganic on a per-pound basis. Additionally, there are a few minor handling issues with the sulphide. Just to recap, you want incredibly low numbers and you’re concerned about your metals. Sulphide should be taken into account.
How about the metals with several valence states? Some of them have circumstances that make treatment more challenging.
There are numerous copper pairs in nickel. Therefore, it’s critical to understand the valence state of several of these metals. It may occasionally be necessary to reduce in. As a result, you can use Chrome as an example. Trivalent chromium is impossible to remove using pH adjustments, softeners, or anything else.
Tribal must first be turned to tribal in Chrome before you may delete or reduce it from it. And in a moment, we’ll talk a little bit more about that. The real estate, however, is something else to pay close attention to.
One of the new metals that is beginning to draw attention is selenium. We’re working with an engineering consulting company that worked on this project for one of our clients as part of their costs. They succeeded by employing so-called zero valent iron. And they succeeded in turning the selenium into selite. Additionally, the only selenium form that can be eliminated is selenite.
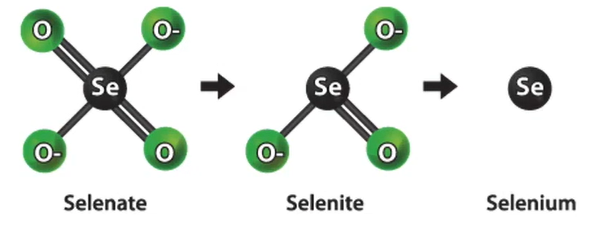
With it, we achieved some fairly good results. Therefore, zero valent iron is kind of a new concept; if you have one in your water, you should think about it.
Molybdenum is a hard metal. And a lot of people utilised molybdate, a highly effective corrosion inhibitor, pretty frequently in our cooling tower treatments when we worked as a water treatment company. But getting out of the water is simply so difficult. really challenging.

Zeolite, which resembles a glue and is covered in these magnetite nanoparticles, can help you remove it. However, depending on the valence state, it can be very challenging to escape. Therefore, if you have molybdenum, you should probably come up with a plan. One possibility is to use evaporation.
What about Chelant
When it comes to electroless, nickel laterals, copper, and other similar materials, EDTA, also known as a sequestering agent, can really make your life miserable. You can discover these substances in your inner plants together with ammonia and phosphorus super phosphate. But once more, these objects are all made to preserve metals, and they are our adversaries.
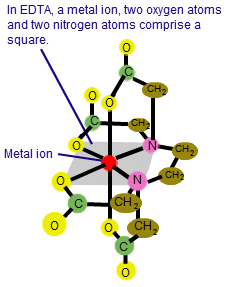
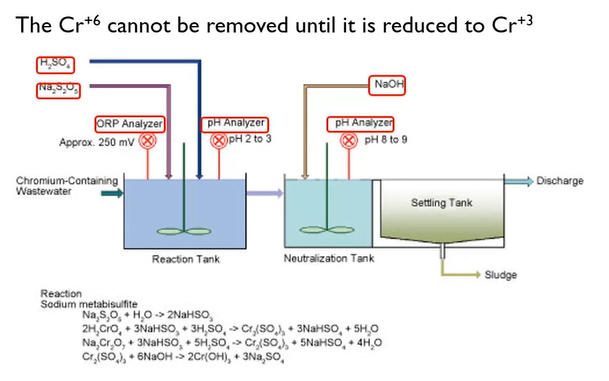
Hexavalent Chrome Treatment
These are intended to maintain metals’ solubility. We briefly discussed the Chrome before. I also said you had to change it from hexavalent to trivalent. In essence, we add bisulfide after lowering the pH to around two or three.
The pH is then increased to between 8 and 9. Because the hexavalent Chrome is yellow, the interaction with that Chrome is somewhat intriguing.
The most crucial point to remember is that you must convert hexavalent chrome to trivalent to obtain it. In a variety of sectors, including plating ones, electroless nickel is growing in popularity. However, the biggest issue we face while working with ammonia is hypophosphate.
And occasionally, you know, folks will just act out while taking baths. They will place a Steel Wall there and use the Steel Wall to play out the remaining metals.
Therefore, a lot of the time, when we have these electroless nickel compounds, people will just ship them off site to be handled. However, if there is sufficient flow, you may sort of bleed them in gradually. And back then, when additional nickel was really expensive it cost around $18 per pound and a nickel was the London metal, you could actually take their sludge and send it in, and they would be paid for it.
Determined the soluble and insoluble metals
It’s somewhat odd for a wastewater scenario, but you may actually make money off the sludge if it contains a lot of nickel or copper.
One of the points I wanted to make clear here is that if my customers experience problems, they should ask themselves whether the metal is soluble or insoluble because the latter is distinct from the former. Among your options is to purchase this Millipore filter system; technically, everything with a diameter of 0.45 microns or above is soluble. That filter would be used to catch these metals that are entering their system.
If you run something through the filter and there is nothing in the filtrate well, you are obviously experiencing a physical issue since the metal that is inside will stay on the filter and the non-metal will pass through. You could not have the proper settlement in your clarifier. Maybe it’s only your solids or two solids in a new clarifier, or maybe it’s just too hefty. Or perhaps you’re experiencing problems with your sludge press. By performing a straightforward solid versus insoluble test, one can discover a completely distinct set of causes. If someone has ever done that, it is none.
Treatment equipment options
Moving on to the equipment now. A mixed media filter is shown below. Do you have any cans? They contain RO systems, hyper filtration, or resin. So, the procedure is straightforward. You possess the tank for equalisation. Not everyone has an equalisation tank, even if you do. Sometimes people will merely use the restroom and a form of equalisation tank, in which case you will treat it using your chemistry. And everything that will occur will take place inside that tank.

You don’t have to worry about anything whizzing through like in a continuous flow system because the reaction is centralised. And most of the time, we use frame filter presses.
You have the most control on the condition you’re trying to address thanks to it. If the pH probe and analyser are functioning, we can usually easily adjust the pH when the flow through the system is greater than 10,000 gallons. Chemicals are metered in, and everything said above is ongoing. I made mention of the seating’s flexibility. It may be difficult to believe all of this.
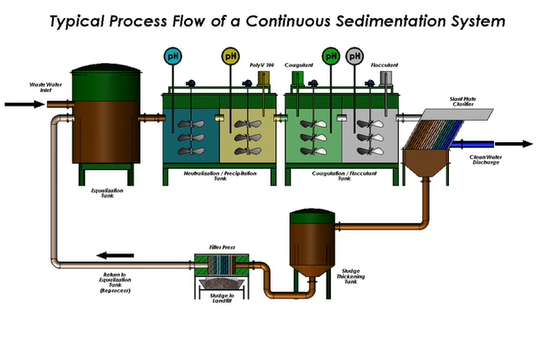
However, there are occasions when very little metal is present in the water. Simply because there is nothing there for things to sort of grip onto, it makes treatment challenging. So, there is a sitting option. This calls for the removal of waste from your sludge thickener and maybe the pumping of it back into your first pH adjustment. Thus, you at least have some solids at your disposal to employ as weight. And because all those metals can sort of touch one another, it’s much simpler.
You should always have time to regulate pH when at home. Again, pH is crucial in the treatment of this form of waste effluent. If I haven’t emphasised it enough, the main takeaway from today is to make sure your pH metres and probes are properly adjusted.
Sand Filter
A sand filter is another option for the back of your clarifier. It works incredibly well to remove any little particles that manage to pass through all the insoluble ones. It’s a good approach to help you handle your problem if you’re hydraulically constrained, have a lot of flow, and your solids in your effluent are fairly high. If they are the CNS backwashed goes back to the front of the system. Whichever else could be oil, getting oil in a sand filter is bad. So, if you have really heavily oil laden waste stream, got to be really careful. Sand filters cannot remove soluble metal. So, what’s going to cause that metal to be soluble?
You will be able to figure that out. But what could cause a metal? There’s a couple of things. What could cause that metal to be soluble?
Ion exchanged resin
Resin that exchanges ions Something to consider if your output is subject to rigorous effluent limitations. You now need to remove specific resins. Some individuals will just really need these to comprehend a little bit, and it’ll be small plants. They do this by dropping down these cylinders.
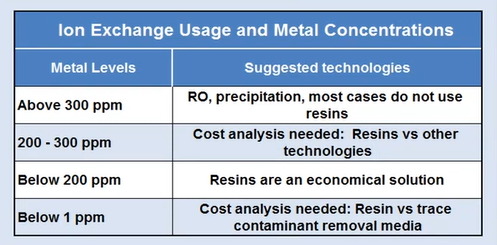
You employ them and fill them with the metal you are attempting to remove. They’ll come in, take them, clean them somewhere else off-site, and then bring them back. However, if you have a really small amount of zinc nickel on something, you might want to use these as a polish.
You may get a sense of where this resin is affordable and where it is simply way too expensive by using the small guide in the lower left corner. Basically, it depends on the amount of metal you’re attempting to remove. But in some regions of the country, they could want to deploy this kind of technology if someone was discharging to a river that had heavy metals.
RO Treatment Equipment
RO is becoming more affordable and is quite popular. Because we have so much water, not as many people are interested in adopting RO for water reuse in the Midwest. However, reverse osmosis has gained a lot of popularity in other parts of the nation for a company in other regions of the nation, you might argue, we might want to reuse our water and a RO could assist to achieve.

Removal of Heavy Metal Ion Using Terminalia Catappa Leaves as Adsorbent
Water is an important source in our daily life, we use water almost all the time, whether for cooking, cleaning, or any other task mostly is related to the usage of water. Hence, there’s no denying that we need water to carry out our daily routine. Therefore, water pollution is something that is very crucial and needs to be taken down.

Effluent from large number of industries such as
- Leather
- Tannery
- Textile pigment and
- Dye pen
- Wood processing
- Petroleum refining
- Photographic film production, etc.
Contribute to water pollution, as it condemned heavy metal.
For your information, this heavy metal is toxic to both human beings and animal.

Furthermore, heavy metal can lead to serious effects such as
- Standard damage to vital organ
- Damage to brain
- Cancer and in some cases
How do we deal with this
There have been many efforts to remove this heavy metal by implementing several methods to remove the substance from our water source to ensure what is clean and is set to use.
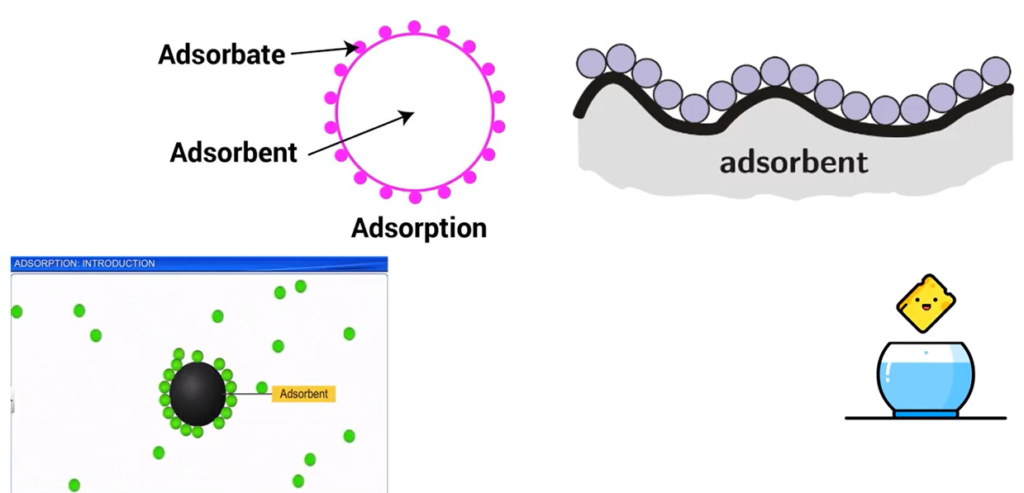
However, removal of heavy metal using a solvent is proven to be the most preferable removal of heavy metal pollution using a solvent to remove this heavy metal component is cheap, not complicated process and very effective. If you are not familiar with what adsorption mean, imagine a sponge, you use it to adsorb water for cleaning. Basically, adsorption is something like that.

The purpose of this part of article is to prove the potential of Terminalia Catappa leaves and adsorbent and to remove the heavy metal like the concept of a sponge. Terminalia Catappa leaves is a very commonly and can be found in a huge amount almost anywhere including Malaysia.
This despite the many advantages of the leave people are still not aware of his true potential, as some still burn the leaves or just ignore it laying on the ground.
A process called adsorption is used to remove the heavy metal using adsorbent. Specifically, then we’re now Terminalia Catappa leaves adsorber. The production of Terminalia Catappa Leave is straightforward.
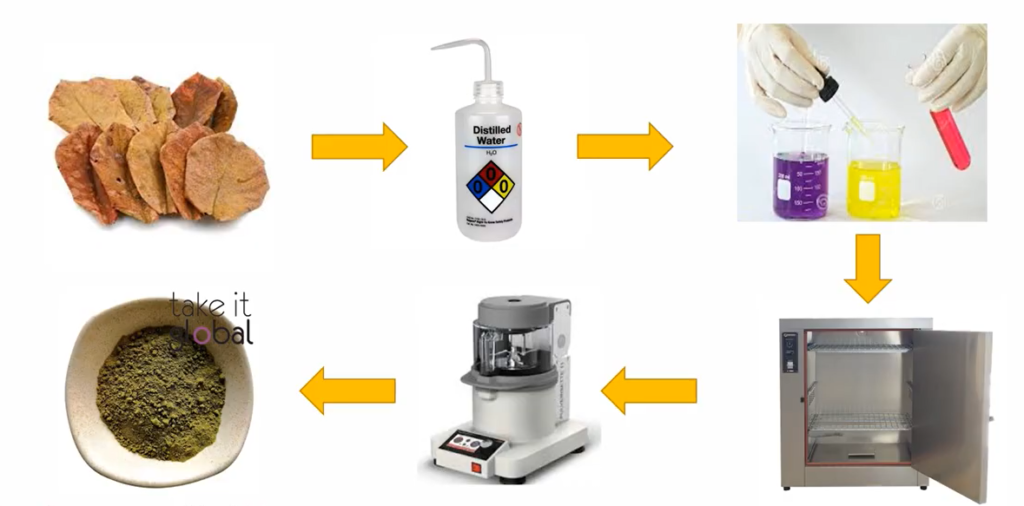
After extraction and cleaning of the sample, and with some addition of chemical grinding and several time drying process, that may Terminalia Catappa leaves in the form of a powder is of time. After that, the effect of parameters such as the
- Time
- Concentration
- Temperature
- pH etc.
On the read of assumption to guess the better series for the process is tested.
An important phase in the analysis of the assumption process is the study of the assumption isotherm and assumption candidate. Our study is significant because employing leaves can assist safeguard and ensure that a water source is clean and safe. This leaf can also be prepared as an adsorbent simply, inexpensively, and without the use of sophisticated machinery. So, I firmly believe that employing Terminalia Catappa leaves to remove heavy metals will both benefit us immensely and aid in the development of wastewater treatment technologies.
Heavy Metal Removal with Adsorption
Now what makes it unique with adsorption media is because it is adsorption, it can’t fit in to grab into a gravity clarifier like we deal with some of our DAF or other mediums. It does need to be pressurised. you would be typically seeing these as a post polishing stage with anything else. So, in some of these projects we’re looking at, we start with a clarifier like a tube settler. And then from there, pick it up with adsorption clarifier.

What is an adsorbent medium not? There are, of course, other things. And we may always look at alternative solutions and methods of doing things. However, organics typically take up organics. You’ll utilise metal-based, granular media when you need to remove metals from water, which is when you’re talking about it.
Aluminum oxide is therefore the most widely used one because it is so cheap. But there are drawbacks. Iron oxide, which is fairly common. We use titanium dioxide as an adsorbent. That is graver’s offering. There are also other people out there. However, these three are the most important participants in the adsorption of metals.
Advantages of adsorbent
The most common one is quite easy to use for metal removal. In fact, all you need to get started is a couple of containers, some media, and possibly some backwashing, depending on the operation. Multiple metals can be removed simultaneously, which is moist, and what’s distinctive is that there isn’t a rollover process like in ion exchangers or GAC with preferences.
Arsenic and uranium are two examples of metals that, once taken in, become bonded to the medium and are impossible to remove, regardless of how many other metals are introduced. Consequently, when a media is actually spent.
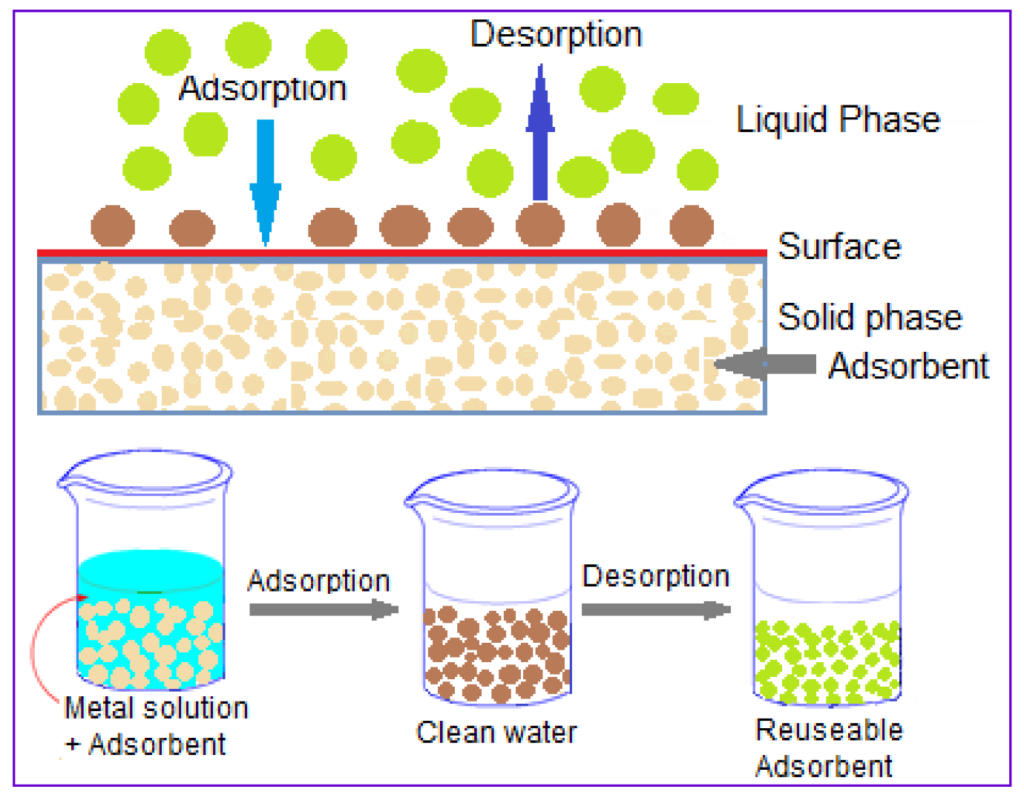
The fact that TDs, chloride, carbonate, and sulphates do not affect the performance of what we are attempting to achieve, which is to take up of metals, is another fantastic advantage that we find when it comes to liking impacted fluids from seawater. Now, that doesn’t mean other factors won’t have an effect on us. And I’ll quickly go through that.
However, TDs in their most basic form typically have little effect on us. Another important aspect of adsorbing medium is that, depending on which one you use, they are typically disposed of in a non-hazardous manner. You can sometimes be a millennial in our media, but it has always been nonhazardous in other media. And returning to basic operation, you can use smaller installations if your water is of the correct grade, you can get much smaller footprints in your treatment systems, which is a big advantage for your customers.
Commonly use of adsorbent
The most frequent application of the product is to reduce metal contaminants from the parts per billion range to a lower parts per billion range.
Usually, when you first start out, you won’t be noticed by the people you’re truly after. 100 parts per billion are examples. If you need to go to drinking water standards and you see this from a mining business or anything similar, this would be the ideal chance. Another example is the most prevalent substance you observe in drinking water, which is 15 parts per billion or less than 10; this is a nice illustration.
Uranium is another; once more, you would pick it up covertly until your media ultimately becomes exhausted, at which point you would replace it. Therefore, it is utilised in Deepwater construction, dewatering, potable systems, and tailings. Due to restrictions that seem to be increasing stricter, you see several situations where various metals must be removed simultaneously.
In one of my projects, the authorities reduced the level of arsenic during the process, necessitating the need for extra treatment. Customers want operations to be straightforward. Another typical use is that. You can often get away without using a chemical. You can set up the media to never be backwashed in specific scenarios if you have extreme regions as you’ll see, mining applications, and minimal water waste.
These are all the metals that I have seen have rules at some point in my life of working with all the many regulations, from surface water discharge to mining, clean-up, and potable drinking. And as you can see on the drinking water, both in the US and Canada, these are common. The main distinction is that people in Canada don’t value uranium as highly as we do. Therefore, you are at a lower level there. However, this is all that adsorption medium should be able to take up all at once, which is crucial.
what do we need to consider?
Parts per million of any substance are likely going to be too high, too expensive, and too difficult to measure unless you have a special circumstance. Unless, once more, there are exceptional situations, such as zero liquid discharge, that force you to employ it sorption. People are all affected by the dewatering of potable water, yet they don’t worry that phosphorus will shorten the media’s shelf life. Iron, manganese, and silica will all have an effect on the media’s quality of life.
However, those can typically be removed by the coagulation and oxidation filtration processes, so they aren’t a problem. Another thing to think about is whether you actually need to backwash or not.

So, then that goes back to zero liquid discharge low liquid use low liquid waste.
What are the priorities for the customer?
We only rinse it for you in places where we sell heavy metals removal, granular, technical terms, and more ramps, as an example. So, you can use the Waterman Engineers Australia product. Without backwashing, the product can be applied.
Both and carbon black are commonly used in sinks and underneath sinks. And we control around 30% of the market for applications involving heavy lead, heavy zinc, nickel, and cobalt.
You are operating in downflow, but all media for adsorbents must be backwashed since during manufacturing, they accumulate particulate and residual waste that must be removed. For other installations, we simply have a method that eliminates all of that; you just install it, perform one or two bed volume forward flushes, and you’re good to go for the duration of the bed.
pH is affected by the metal. However, this often operates within the confines of acceptable water quality. Therefore, the pH affects certain metals’ 6.5-8.5 ranges. Arsenic prefers a lower pH, while lead prefers a higher one. The lower the pH, the better the outcome. It will function down to four. With a pH of four on arsenic, it will shoot through the roof in the capacity.
However, there are some metals that can’t be removed and there are toxicity and leaching tests. Additionally, what you’re taking up may have an affect on whether it passes; activated alumina doesn’t pass as frequently as iron media. And we always succeed since titanium dioxide sticks to metals considerably more firmly than other media.
Because seawater contains more than simply salt, there are interferences. Not only sodium chloride is involved. Presents are other additional metals there that might cause problems. Strontium is a nice example. Therefore, you need to review the application. Additionally, you must be clear about what you want to say. what the restrictions are. It goes without saying that the boundaries are crucial. However, we have utilised it in seawater in the past.
Performance won’t be harmed. However, since the titanium dioxide is being bound together for us by an organic binder, we advise limiting the chlorine to less than 0.5 parts per million. Therefore, you only maintain low levels of ferric oxide and activated alumina at 0.5 ppm. Pouring is not a concern for you.
Filtration is a common pre-treatment method. And because they are so simple to absorb, it is iron and manganese. Nevertheless, they will have a significant influence on life. Therefore, you should consume as much iron and manganese as you can. Is it often the most pre-treatment you’ll perform?
Organics will cling to and coat the medium, depending on whether they are lignans, tannins, or bigger organics. Naturally, that will cause it to go blind. Therefore, you must be aware that the typical amounts of one to two parts per million are of no great concern. However, if you’re coming from a stream that was formerly a dump, you should be careful because there are some awful things there, like heavy organics and long-chain molecules that will coat you. And you can obtain bio growth. It doesn’t happen very often, although certain bacteria do desire to survive on it.
If there is no preference, one metal will always have a different capacity from another metal when it comes to adsorption media. Absolutely, that is the case. Therefore, we are unable to delete one before the others. Is this accurate? That is accurate. One metal will always exhaust before the other if you’re removing more of it or more than one metal.
Using industrial and agricultural wastes as adsorbents to remove heavy metals
The purpose of this part is to look into the usage of less expensive adsorbent for heavy metal removal from wastewater. One of the most expensive and three-valent metals used in industry is copper. The wastewater can be taken using a variety of methods, such as adsorption, membrane filtration, cementation, and electro dialysis.
This section focuses on the extraction process utilising rice husk and fly ash. Many different sectors, like metal plating, mining, etc., produce aqueous waste that is contaminated with heavy metals. Carbon is still an expensive element, despite being used extensively in water and wastewater treatment processes. The necessity for a safe and affordable way to remove heavy metals from contaminated water has necessitated study in recent years. Low-Cost agricultural waste by-products such as sugar cane buggers.
Problem Statement
Basically, heavy metals are one of the most persistent pollutants in wastewater due to the discharge of significant amounts of metals polluted in wastewater, including carrying heavy metals like copper, phosphorous, nickel, etc. are the most dangerous among the chemical intensive sectors. Therefore, it is not required to remediate metal-contaminated wastewater before releasing it into the environment; doing so would cause the aquatic ecosystem to perish.
Even the large concentrations of heavy metals in the soil decreased the quality and quantity of the food by preventing plants from absorbing other nutrients and hindering plant growth. Therefore, the problem of the heavy metal chain is basically present everywhere, especially in the industry. They need to remove the various types of toxic metals present in the effluent water otherwise it will only affect our environment in which we are living and aquatic system plants, animals, especially us humans, because we humans depend on these three fundamental things to survive.
What does solution do?
Therefore, the main course of action is to remove heavy metals from wastewater and employ industrial and agricultural waste as adsorbent. The adsorbent which we are using, it will adsorb the toxic metals from the wastewater. The adsorbent, which we are using is totally low cost economically eco friendly and very beneficial to small scale industry those who can’t afford third degree treatment, it will not use highly cost-effective machines like ion exchange membrane filtration, electro dialysis etc.
Beneficially for the saving electricity, it is used for the saving electricity as compared to the heavy machines and easy maintenance as compared to the large machines.
Procedures of heavy metal removal
We are discussing the steps that must come before rice husking. To increase the specific surface of rice husk, we activate the husk using this procedure. With the aid of a signal, the rice husk was saved with a particle size smaller than or equal to one millilitre and transcribed for a subsequent experiment. This Rice Husk was blended with one molar of the ingredient or solution for 24 hours at room temperature while stirring every 500 metres at a speed of 150 RPM.

Thus, the reagents had completely adsorbed themselves to the raw material. Following this assertion, the modified Rice Husk was filtered and repeatedly washed with distilled water until the pH value remained constant. This adsorbent was then dried at 110 degrees Celsius for all of it before the attribute-rated Rice Husk were opened. Then we made a synthetic copper solution with the help of a copper sulphate. After then we add dried Rice Husk into the copper solution and maintain the pH of five. Then after we make the solution at 200 RPM with the help of mechanical stirrer during mixing, we provide the contact time of 120 minutes and adsorption dose as further 20 grammes per litre and we get the best copper removal. The copper removal with the help of fly ash is Same as the remote process of using Rice Husk.
Result of heavy metal removal by Rice Husk or fly ash
We take an adsorbent dose starting from 20 and goes up to n 70 with the increment of 10 percent, and we see that at the adsorbent dose of 60 we get a better result according from that other dose, even in Rice Husk and fly ash. In Rice Husk we get 0.99 milligrams per litre copper removal whereas in pliers, we get zero-point 79 removal length then and the best part of this is that it is very cheap, low cost, eco friendly agricultural waste, which is Rice Husk and fly ash, which is industrial waste, which we get easily available. Then after we come to the part of graphical comparison, here basically shown the graph of from 20 milligrams to 60 milligrams of the adsorbent doses and their effects and the doses we have taken which I mentioned of removal percentage.
Conclusion of heavy metal removal
Finally, we reach a conclusion. In conclusion, the findings demonstrate that the least expensive adsorbent can be successfully employed to remove heavy metals at concentrations between 20 and 60 milligrams per litre. At a 10% removal rate, it was discovered that the amount of inexpensive adsorbing and the concentration of adsorbing were related.
It was discovered that two hours of contact time were required for maximum adsorption, and that a pH range of six to seven was ideal for heavy metal absorption. Finally, we can state that this agricultural waste makes it simple to remove copper.
What is Chromium VI and How it is removed from water?
Wastewater from industries contains harmful pollutants, including chemicals and heavy metals. If this wastewater is released into the environment, without removing these pollutants, it ends up contaminating aquatic bodies like ponds and rivers and groundwater. Hence, scientists are finding efficient ways to treat industrial wastewater. One such pollutant in wastewater is chromium VI.
It is a heavy metal that adversely affects human health. It increases the risk of cancer causes skin allergies and damages the liver and kidney. To remove chromium VI from industrial waste, it is reported that water plant can be used to tackle this pollutant.
Chromium VI is a human carcinogen, and it is produced in the waste from electroplating industries, telling industries, then wood preservation as well as textile manufacturing industries.

To show that water hyacinth can be used to remove chromium VI pollutant from water they collected water hyacinth and plants growing on water bodies and dried them overnight at 120 degrees Celsius. Then the ground is dried plants to make a fine powder and sifted through a point three-millimetre mesh.
When this powder was mixed with base water, it could absorb more than 60% of chromium VI from wastewater in 30 minutes time. This method of removing chromium VI is low cost and easy to perform.
Water hyacinth can efficiently remove chromium VI from the wastewater at a pH of two which is the acidic pH then 30 minutes time is required to efficiently remove the Chromium VI from the wastewater, it works better on the room temperature, it works efficiently at a very minimum dose of 0.4-minute milligrams per 100 ml of wastewater. So, these were the outcomes of the study.
water hyacinth is scientifically known as Eichhornia Chrysopsis. It grows at a very fast rate and covers water bodies in a very small span of time. This reduces the amount of oxygen in the water and less sunlight can penetrate the water, hence, it negatively impacts aquatic life. Now, scientists have put this invasive plant species to good use. Since water hyacinth powder is low cost efficient, easily available, and convenient to use. It will be a huge aid in water remediation programmes. Treating industrial wastewater with heightened powder before releasing it into the environment can save many water bodies from deteriorate.
Lead in drinking water
The third question in the series of common queries concerning water and wastewater treatment is covered in this section of the article. What is the highest acceptable level of lead in drinking water, was the inquiry. There were 11% who chose the initial option of 0.09 milligrams per litre, 76% who chose 0.05 milligrams per litre, 9% who chose 0.01 milligrams per litre, and 5% who chose one milligram per litre.
- The effect of lead in drinking water
- Source of lead in drinking water and
- How can we reduce lead in drinking water.
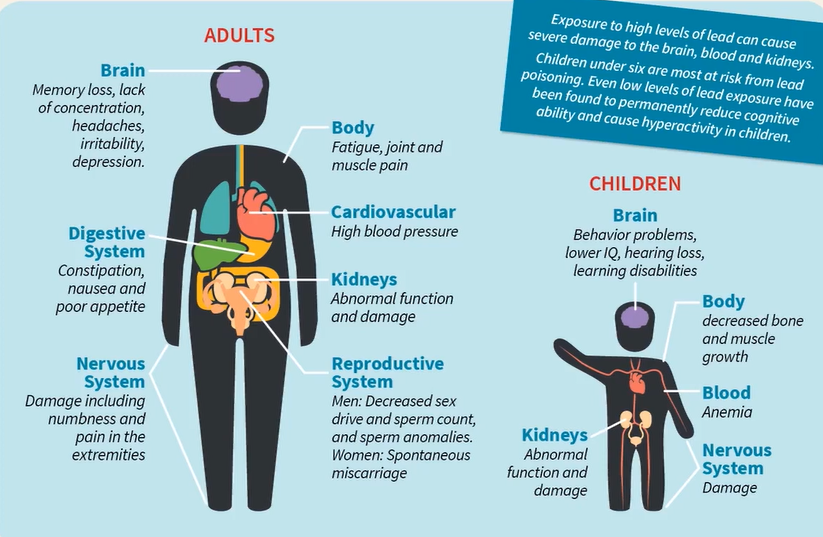
Here we can see in this image, lead can affect
- Brain
- Digestive system
- Nervous system
- Body cardiovascular
- Kidneys and reproductive system in adults and
- Behaviour problem
- Lower IQ
- Hearing loss
- Learning disability
- Kidney’s problem
- Decreased bone and muscles growth
- Nervous system problems in children.
This graphic demonstrates how exposure to high levels of lead can seriously harm the kidneys, blood, and brain. Lead poisoning poses its greatest risk to children under six. Children’s hyperactivity and cognitive ability have both been demonstrated to be permanently reduced by even low levels of lead exposure.
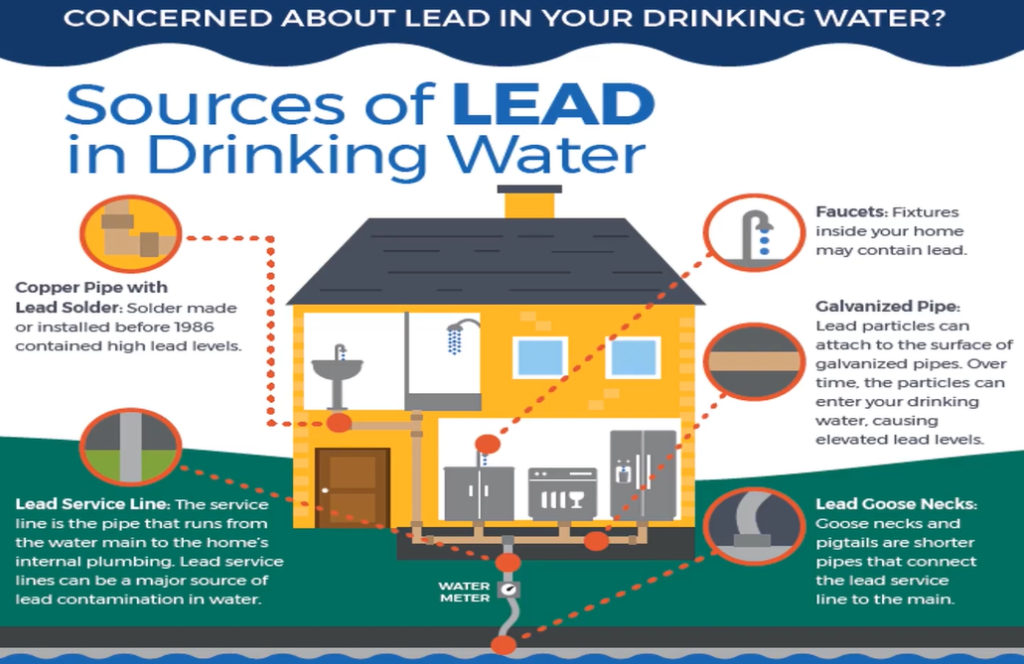
As per the Environmental Protection Agency, there are many sources of lead.
- Copper pipe with lead shoulder,
- Lead service line,
- Pockets galvanised pipe,
- Lead gooseneck and
- Water metres are main sources of lead in drinking water.
The pipe that connects the water mains to the interior plumbing of the residence is known as the service line, and it was manufactured or installed before to 1986. Water faucets inside your home might contain lead, and lead service lines can be a significant source of lead poisoning.
Pigtails, which are shorter pipes that connect lead service lines to the mains, and water metres are the main sources of lead in drinking water. Lead particles can stick to the surface of galvanised pipe in time and enter your drinking water, creating excessive lead levels.
What can we do?
There are a few techniques to lessen or get rid of lead in drinking water. Therefore, flesh comes first. if water has not been used in the house for a few hours, such as while leaving for work or arriving home, or for five minutes in any bathroom pocket. Before drinking or cooking with the water in your home, you can also flush the plumbing by running the dishwasher, taking a shower, or doing a load of laundry.
For drinking, cooking, and preparing infant formula, only use cold water. Lead is not removed from water by boiling.
Other thing is filter for drinking and cooking Use a filter that is NSF certified to remove lead especially if you are pregnant or have children under age six and last thing is maintenance. Replace the filter cartridge in your pocket aerator and clean it frequently add manufacturer’s recommended schedule and consider replacing pockets time to time. As per the BIS the limit is 0.05 milligrams per litre and as per EPA it is 0.01 milligrams per litre.
Phosphorus Removal to Achieve Environmental Compliance At least Cost
Wastewater Treatment Works upgrades are predominantly going to based around the findings of the water industry National Environment Programme, when it which has created new or updated phosphorus consents being applied to wastewater treatment works throughout the country.

Now these are from sites as low as 100 population equivalents to sites in excess of 1 million population improvements are very broad range. And phosphorus is ubiquitous in our wastewater being used in laundry soap and toothpaste and even prevent plumper solvency and drinking water. anthropogenic sources account for over 40% of phosphorus discharged into receiving watercourses.
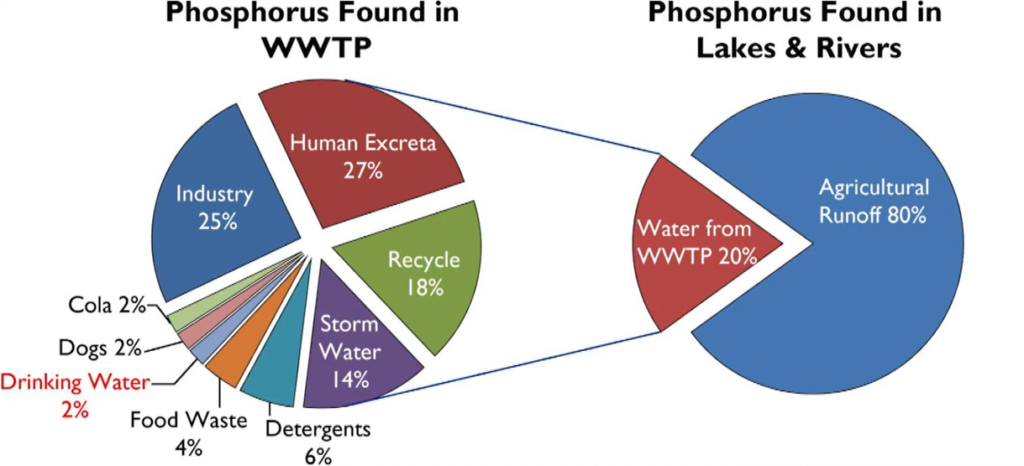
The result being that the wastewater treatment works becomes a point source discharge of phosphorus unless it’s removed on site.
There are two main techniques to remove phosphorus
- Biological Phosphorus Removal via EBPR and Enhanced Biological Phosphorus Removal are essentially.
- BNR biological nutrient removal
The alternative is the dosing of chemicals to precipitate out the phosphorus.
Chemical Phosphorus Removal
Now, whilst each has their own advantages and disadvantages, the purpose of this part of article will focus on chemical precipitation. So, chemical Phosphorus Removal generally relies upon the reaction between a trivalent metal ion, typically iron in the form of iron sulphate or iron chloride or aluminium in the form of alum or poly aluminium chloride with ortho phosphate within the water streams, forming an insoluble metal phosphate.
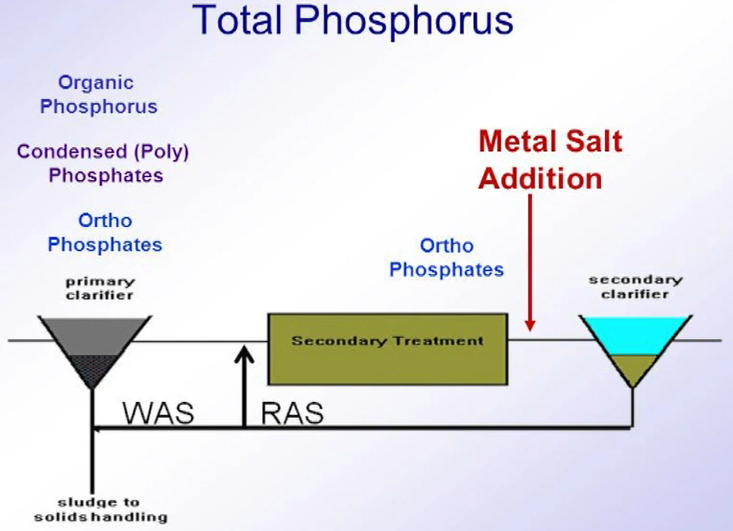
Although now while alternative chemicals are available, including magnesium, calcium, and more recently lanthanum These are typically not in common use. Phosphorus from anthropogenic sources can be broken down into three main categories
- Reactive ortho phosphorus
- Polyphosphates and
- Organic phosphates
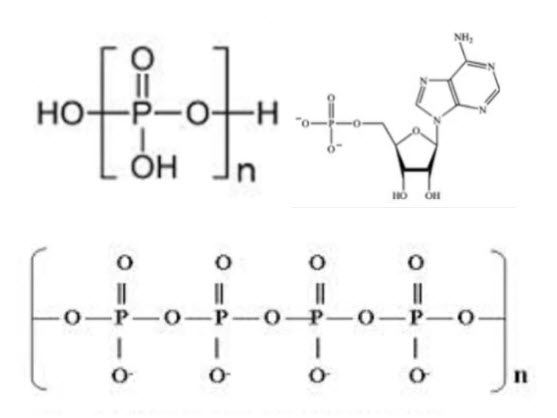
which only the reactive ortho phosphate can react with the metal ions. polyphosphates can be broken down through biological wastewater treatment can become available for chemical removal at later stages in the wastewater treatment process.
Whilst organic phosphates are more complex and difficult to remove. On a stoichiometric, that’s a chemical balance basis, the reaction should occur on a one-to-one molar ratio between metal ion and phosphorus.
However, since each wastewater at each site is a cocktail of compounds discharged into the catchment, each site wastewater will have its own individual characteristic as well as other factors. For example, retention times within the upstream sewers will affect chemistry within the water through again for example, the presence of sulphide from septic city and for that reason, the real-world requirements will differ from the theoretical Stokely metric balance.
Excess dosing of metal salts may have negative impact upon the wastewater treatment process, since it removes alkalinity which is also required for nitrification that’s ammonia removal. Now, jar testing as a technique facilitates the rapid evaluation for metal salt effectively required to remove phosphorus from a wastewater stream.
The technique is inexpensive, and samples from different areas of sites can be tested both in parallel with each other and with different chemicals, as well as different doses of the same chemical. Furthermore, since the test is upon reasonably sized samples, analytical suites from the treated water can be evaluated as required, with a range of analytical determinations including cod alkalinity, total suspended solids, phosphorus, of course, even free iron. And, if you’re quite clever about it, sediment levels can be measured to evaluate the amount of sludge produced on site.
When you’re testing a single bulk sample is used, which is then split into several jars, as well as from each of the different doses of chemical applied to the jar. During a short as two-minute rapid mixing phase, the samples are rapidly mixed, then allowed to flocculate a gentler mixing speed for about 30 minutes, and then settled, after which the supernatant is decanted and collected for analysis.

Sludge levels are recorded at this point in order to evaluate the additional stage make Waterman Engineers Australia undertaking these tests for many clients in the industrial municipal sectors, facilitating cross checking actual site performance against lab data for the optimization of sites or even facilitating the most cost-effective chemicals to dose as well as the dose requirements. For one client. We did this across 20 sites, which included the development of bespoke methods and calculations through which the generated sludge make, and volume associated with chemical removal could be assessed.
To summarise if chemical dosing for phosphorus is your preferred option for P removal, ensure that the dosing requirements are thoroughly evaluated through jar testing before proceeding with design.
Wastewater treatment from phosphorus with electromagnetic nano-mill
Phosphate-containing chemical wastewater poses a risk to the environment. The two methods of phosphate removal chemical removal using different chemicals and sorption are used by the majority of facilities. Purification is ineffective during the production process. Decontamination in reactors can take up to 30 minutes because it must be done in phases and requires a lot of time.

We investigated how to remove phosphates from wastewater using an ABS vortex layer magnetic mill. We looked at how treatment time and medium pH affected the degree of decontamination, and we calculated the ideal ratio of ration components to determine the mill’s processing rate as well as the ideal process conditions.
Industrial wastewater with a phosphate concentration of 4500 milligrams per litre and a pH of 4.0 L 10% was the subject of the study. In the mill regime, lamb milk was processed for one to three seconds while being consumed at a rate of 70% to 120% sticky metric. During processing, the pH of the effluent was between 4.0 and 12.0. According to test results, phosphate was used to determine the amount of phosphate in wastewater.
Utilizing a pilot unit where well-prepared lamb milk was provided to a consumption tank and effluent from the production process was forced to apply to equalise, the research was carried out under industrial settings. According to their investigation into the decontamination of wastewater in the ABS, a magnetic mill is more effective than the machinery now in use for this purpose.
Phosphates are transformed into water-soluble molecules in a single step that takes one to three seconds. Using the ABS for wastewater decontamination allows us to reduce the consumption of reagents in power, decreases equipment footprint, and enhances the quality of the decontamination process. The reaction employed is lime with five to ten percent extra calcium oxide of the theoretically required quantity.

Equalized wastewater and lime milk from the consumption tank are pumped straight into the ABS since the process in the ABS mill only requires one step. In the ABS, both components are thoroughly blended, distributed, and electromagnetic field-exposed before being pumped to settling tanks for clarity. pH metres are used to manage the wastewater’s acidity while flow metres track the flow rate of wastewater and lime milk. The study made it possible to determine the best circumstances and levels of efficacy for wastewater treatment.
Nitrogen Removal in Municipal Wastewater
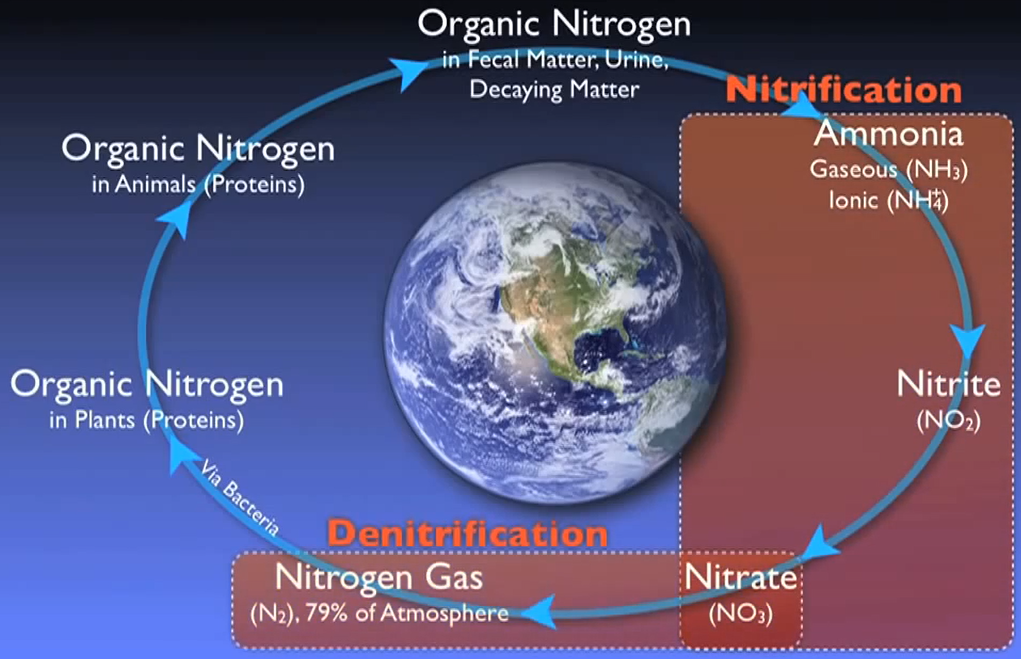
Nitrogen follows a fundamental cycle, just like carbon does. All living things that have ever lived or will ever exist on Earth depend on this cycle. Wastewater treatment facilities are essential for restoring nitrogen into the cycle with minimal negative effects on the environment.
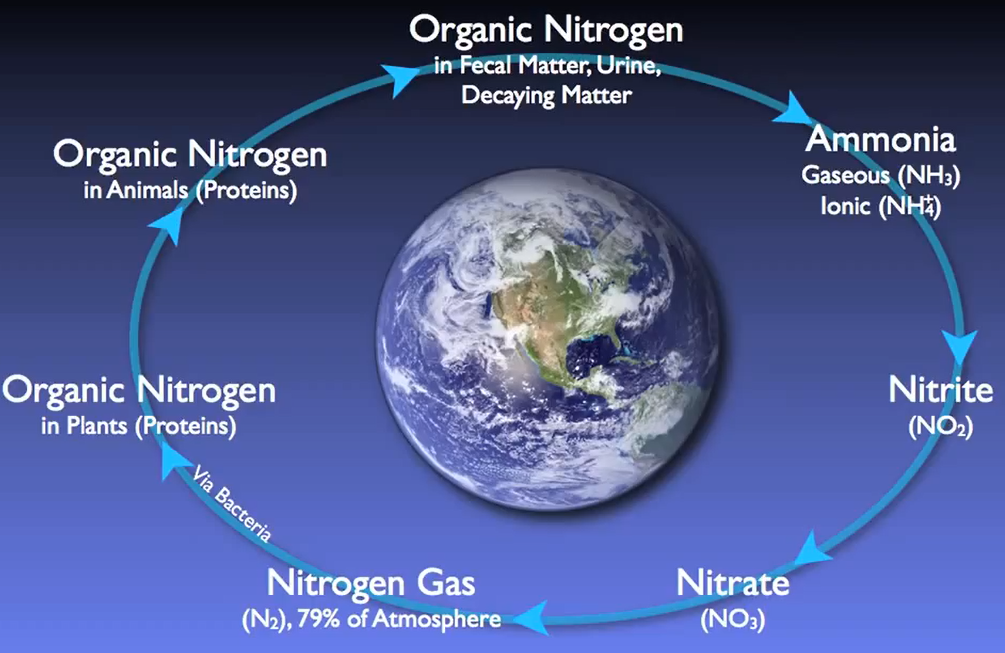
For local plant, animal, and human populations, high quantities of nitrogen entering natural streams can lead to hazardous conditions for wildlife, dissolved oxygen depletion, and excessive algae development.

Nitrogen in Water
Numerous methods allow nitrogen to enter wastewater. Urea is the main source of nitrogen in normal municipal effluent. Urine, other nitrogen-containing substances, food processing waste, chemical cleaning products, and several other industrial elements. Wastewater contains nitrogen in a variety of forms that have been grouped into distinct main types.
- Ammonia nitrogen is nitrogen that has taken the form of ammonia.
- Nitrogen nitrite is also present.
- At a wastewater treatment facility, nitrate nitrogen is typically created during the actual biological processes.
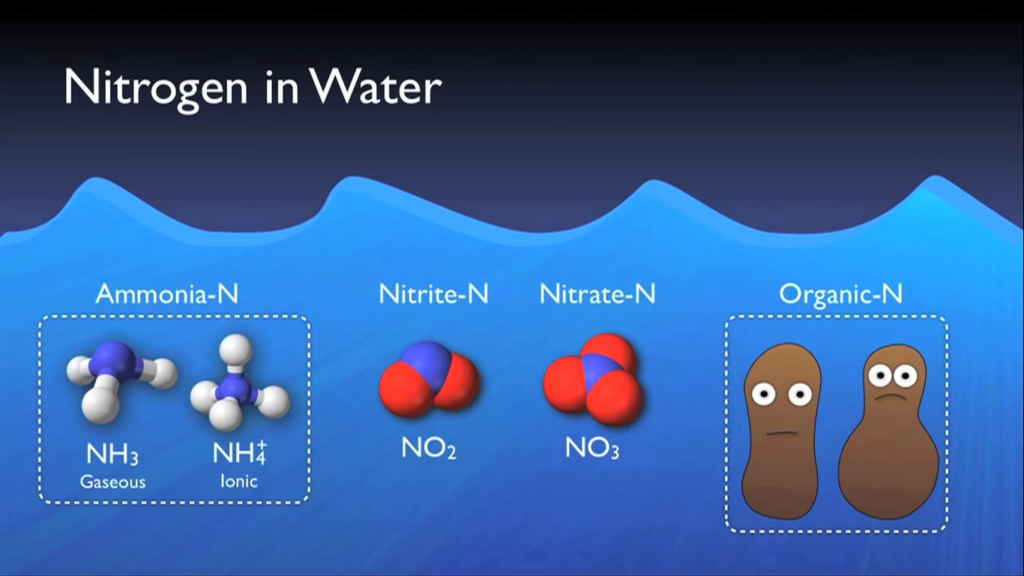
In addition to these forms, nitrogen and other dissolved organic molecules make up a minor portion of the cell mass of the organisms in the system. This category is known as organic nitrogen, and it normally contains a specific quantity that cannot be eliminated by the biological procedures that will be discussed in this section of the text. Total inorganic nitrogen, or TIN, is simply total nitrogen less organic nitrogen, as the name implies.
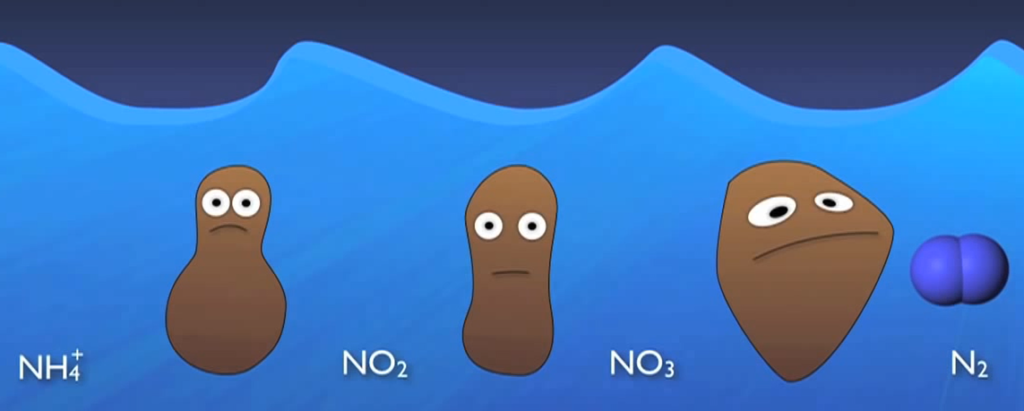
Simply put, nitrogen is flushed, washed, or otherwise injected into the sewer system in numerous forms. For instance, most of this organic nitrogen urea is promptly hydrolysed into ammonia. In the case of water gaseous ammonia, NH3 is largely converted to ionised ammonia or ammonium, which is subsequently transformed by NH4+ specialised autotrophic bacteria or nitro fires into nitrite and O2 and ultimately nitrate and NO3 by various biological processes.
As the amount of dissolved oxygen is decreased by the Nitro fires and other organisms in the basin. Other specialised heterotrophic bacteria known as de nitro flames can survive by using the oxygen attached to the nitrate molecules for respiration and creating nitrogen gas as a by-product. When this happens, the nitrogen gas bubbles up out of the water and into the air.
Returning to the process’s commencement, let’s examine each of these critical steps in more detail.
First, the majority of the organic nitrogen instantly hydrolyses into ammonia as a result of a natural reaction in an aqueous solution or in water.
The pH of the water has a significant impact on the balance between the gaseous and ionic forms of ammonia, the majority of which changes automatically to the ionic form ammonium. A more basic solution will favour the gaseous form of ammonia, while an acidic solution will favour the ionic form of ammonium. Nearly 90% of the ammonia will be in the ionic form because wastewater normally has a pH between 6 and 9.
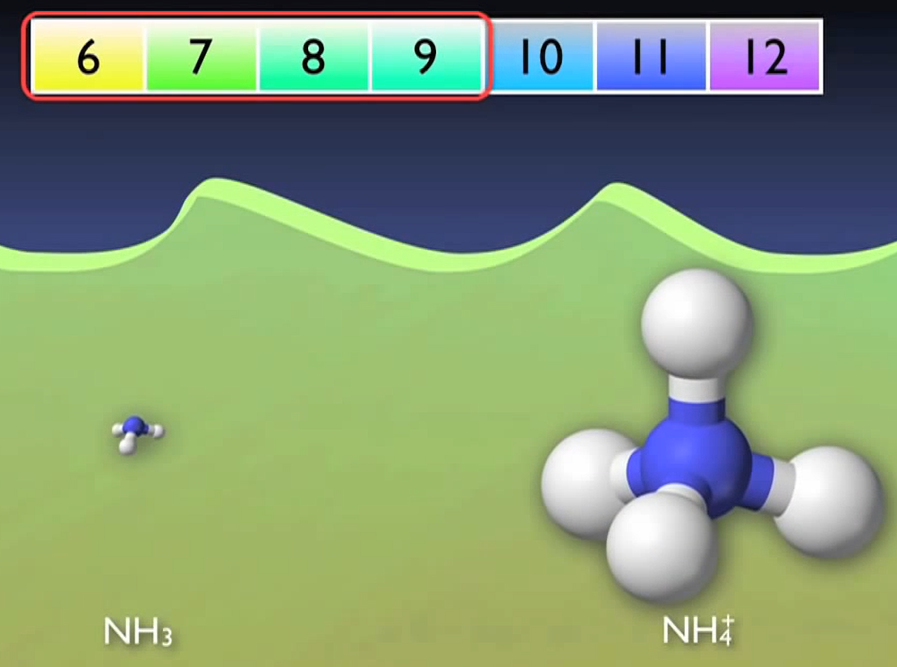
It is not required to worry about each type separately because ammonia testing takes both the ionic and gaseous forms into consideration. This conversion can occur without the use of microorganisms. It simply occurs.
Ammonium is transformed into nitrite and subsequently nitrate as the process’s following stage. Usually referred to as “nitrification,” this two-step process is made easier by a specialised autotrophic bacterium.
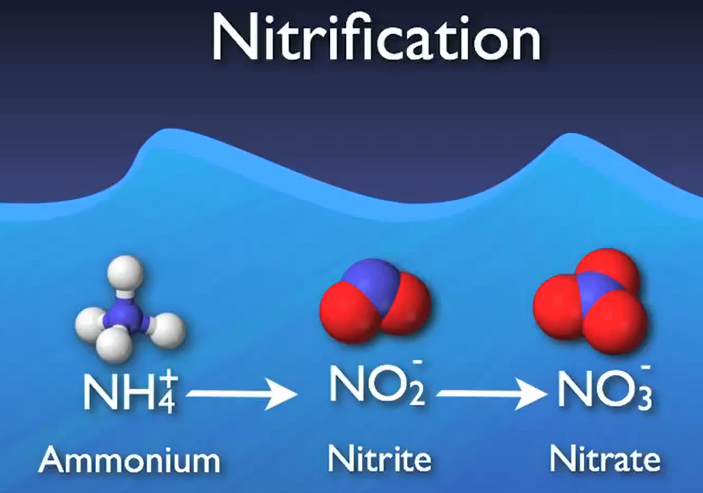
What does autotrophic actually mean then? When it comes to how organisms receive the carbon they need for growth, there are two main categories: autotrophs and heterotrophs. Alkaline bicarbonate plants are excellent illustrations of how autotrophs can receive their carbon from inorganic sources such as carbon dioxide.
On the other hand, heterotrophs need carbon from organic sources. In essence, heterotrophs obtain carbon by devouring other organic substances; humans and other animals are prime examples of this.
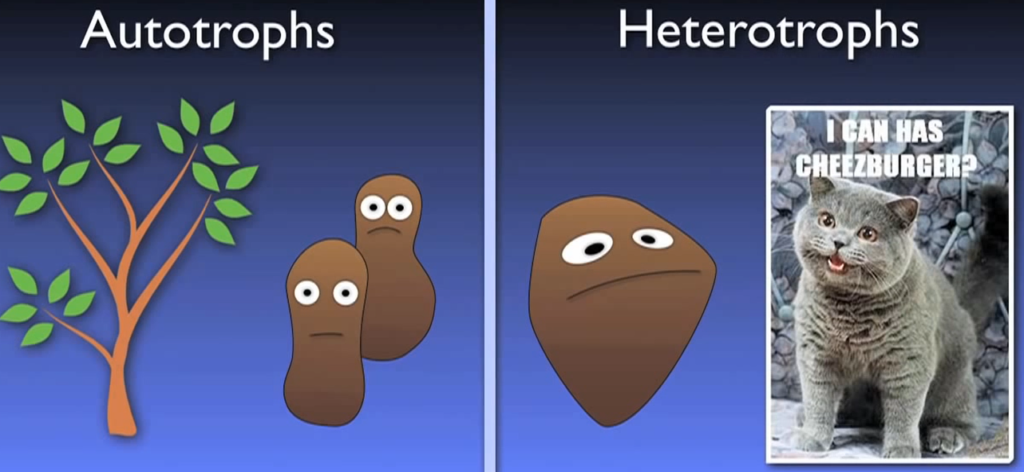
As opposed to bacteria that consume BOD heterotrophically In a biological wastewater treatment system, autotroph nitro flames require more time to mature and maintain their population. The SRT and nitrogen reducing plants are determined by the nitro fires.
The temperature of the wastewater and the level of dissolved oxygen determine how quickly nitro fires grow. You run the danger of unintentionally wiping out nitro flames and losing nitrification if they aren’t given enough time to flourish.

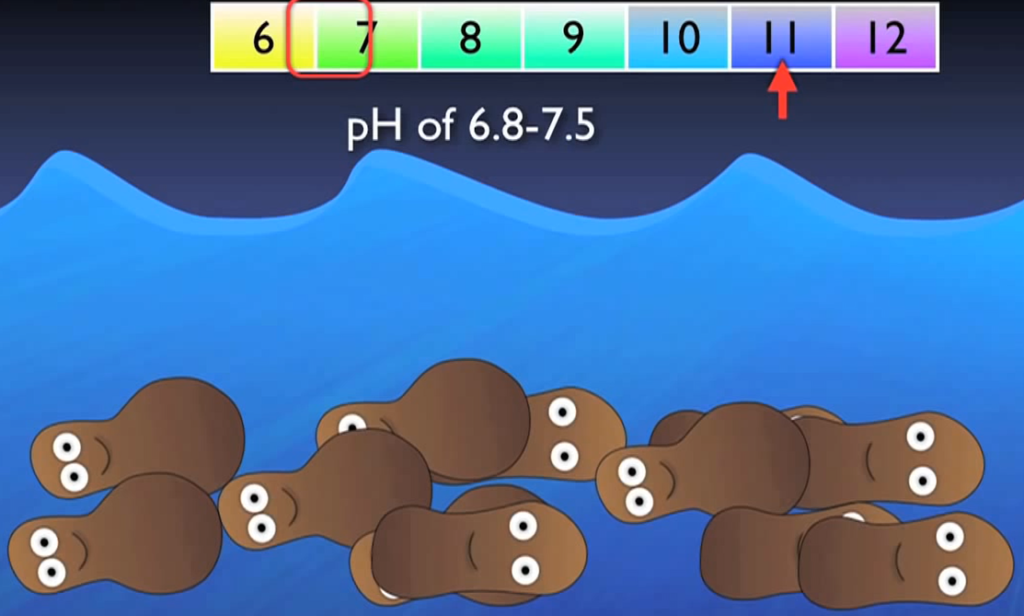
Faster growth results from higher temperatures and DO concentrations. Slower growth results from lower DO concentrations and cooler temperatures. Additionally, nitro fires might go completely dormant when there is no DO around. It’s vital to keep in mind that nitro flames are also quite sensitive to pH, typically operating most effectively in water with a pH of 6.8 to 7.5.
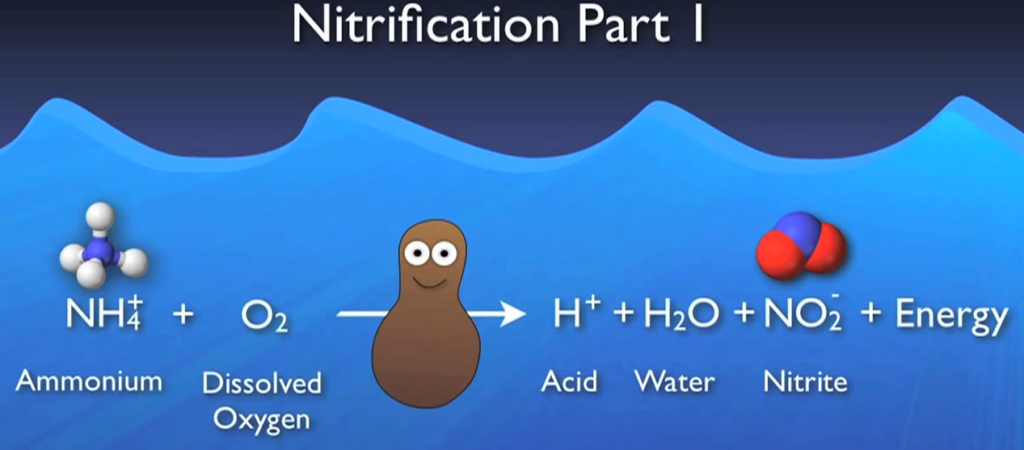
Once a healthy population of nitro fires has grown within your system, the first phase is primarily carried out by a colony of ammonia-producing bugs. Ammonium and DO will be used by oxidizers to produce acid, water, nitrite, and energy. The water produced is absorbed in the system, the nitrate moves on to the next step, and the energy is used by the bacteria to grow and multiply. If your system doesn’t have enough alkalinity, the acid produced here could create inhospitable acidic conditions for the bacteria, leading to major processes including loss of nitrification.
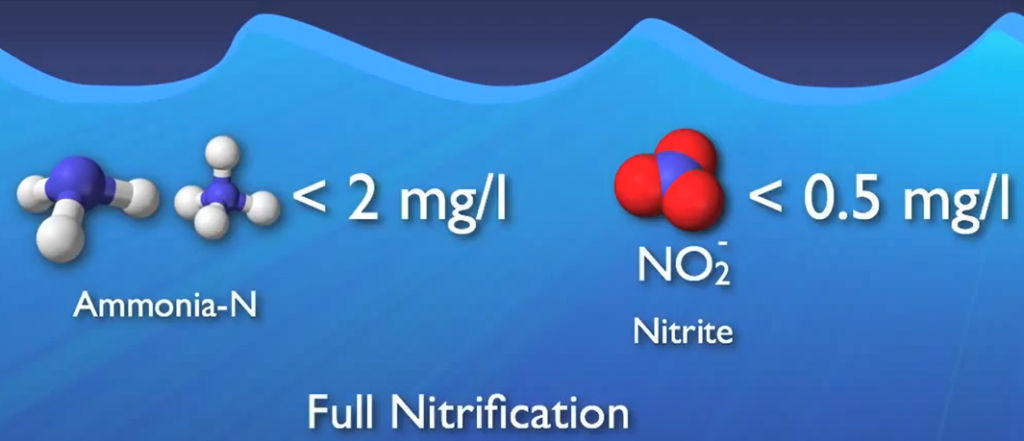
This nitrite and more DO are used in the second step, which is primarily carried out by a group of microbes known as nitrite oxidizers, to transform it into nitrate. Nitrification is the process of changing ammonia into nitrate. When ammonia concentrations are decreased to less than two milligrams per litre and nitride concentrations to less than half a milligrams me per litre, complete nitrification is typically seen.
Nitrification is a particularly oxygen-hungry process, as was already established. In contrast, 1.2 pounds of oxygen are needed to eliminate one pound of BOD. However, 4.6 pounds of oxygen are needed to convert one pound of ammonia to nitrate. because adding dissolved oxygen to wastewater requires a lot of energy. Every treatment plant’s electrical expense would benefit if they didn’t aerate more than necessary. Over aerating is a complete waste of money because it has no positive effect on the process.

Nitrogen is still present in the system after nitrification, primarily in the form of nitrate and as a component of the bugs. Despite not being as harmful as ammonia, nitrates can still contribute to eutrophication and, if released into a source that supplies drinking water, they can put human populations in danger, especially young children, by preventing blood from oxygenating properly, a condition known as Blue Baby Syndrome.
De-nitrification is the ultimate process that eliminates nitrogen from the system. One may assume that de-nitrification is just the opposite of nitrification, which is the conversion of ammonia to nitrate. Fortunately, this is not the case, which is confusing. A specialised heterotrophic bacterium does de nitrification. To complete this phase, these people need some unique circumstances.
To begin with, they require food or BOD to oxidise that food. They also require oxygen and DO is their preferred and easiest source of oxygen. However, if there isn’t enough DO available, they turn to alternate sources like nitrate since they can extract the oxygen from nitrate molecules to meet their needs.
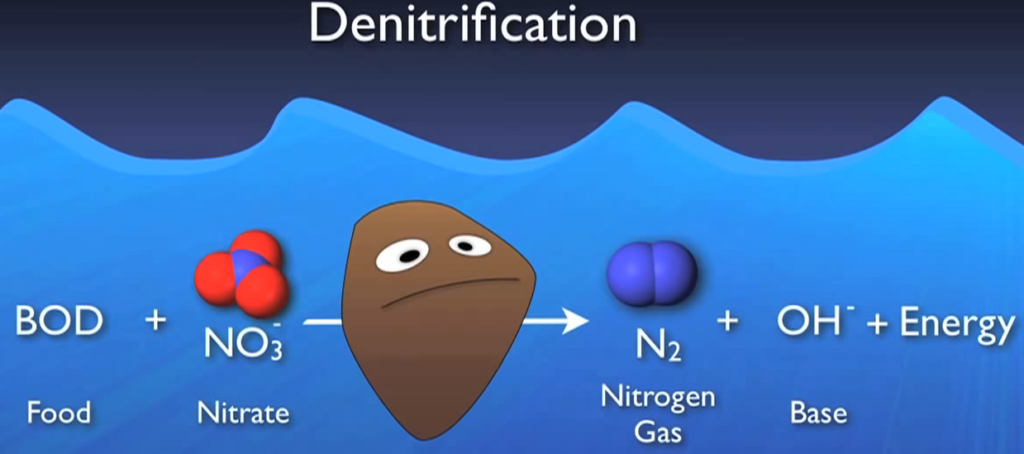
Denitrification is critically necessary in this crucial environment, where DO is absent yet nitrates are considered anoxic. These bugs use nitrate and BOD to create nitrogen gas and energy base. Actually, the base serves as a highly effective buffer for the acid created during nitrification, the nitrogen gas, which floats to the surface and releases tiny bubbles into the atmosphere.
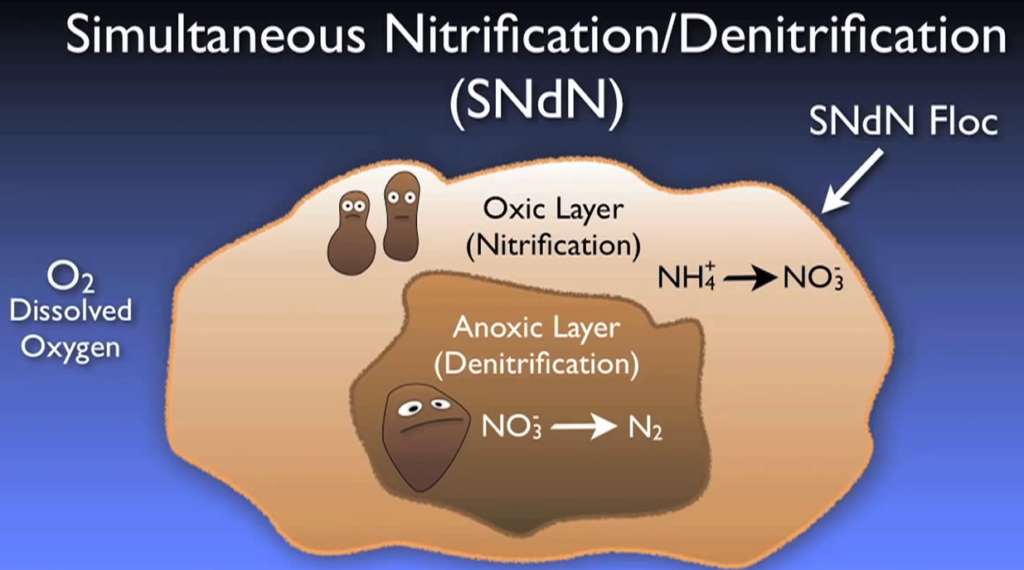
Despite being beneficial in the biological basin, nitrification in a clarifier may produce unwanted floating muck. Alternative methods of creating anoxic conditions abound. Some treatment facilities aim to create these anoxic zones inside the oxidation ditch or aeration zone by strictly managing aeration or by turning off aerators for predetermined periods of time, while others are built to have designated anoxic zone volumes in the flowchart.

Additionally, simultaneous nitrification and denitrification can occur when micro anoxic zones form inside the bacterial flow itself under specific circumstances. Depending on the local discharge permit, different denitrification levels can be necessary. But the effluent stream typically contains only one to three micrograms of nitrate after full biological denitrification.
Problem Solving
Let’s now examine some actual operating circumstances. You observe that the ammonia level in the effluent is starting to exceed one to two milligrams per litre during your routine operational sampling. Let’s consider the potential causes of this; after all, nitrification is an oxygen-hungry process. Perhaps there isn’t enough aeration. Nitro fires are also known to burn more slowly. The SRT may be overly short because of temperature. Additionally, we are aware that nitro fires are extremely sensitive to pH shifts and hazardous environments. Perhaps a disturbance of that kind is the cause of this performance decline. Knowing these things makes it simple to identify potential remedies.

If low DO is the problem, adding aeration will restore full nitrification. To keep a healthy nitrifying population, the SRT may need to be raised if temperatures have fallen. Depending on the precise cause, various actions may be required if pH or toxicity is to blame.
The second issue is that you detect an increase in nitrate concentration in your effluent rather than ammonia. What might be the reason behind this? We are aware that the formation of anoxic conditions in the basin is necessary for de nitrification. Over aeration may be preventing the development of these circumstances, although a less likely but still plausible explanation would be influent BOD levels that are lower than normal. If excessive DO is preventing the development of anoxic conditions, reducing aeration will aid in re-establishing these unique circumstances. There might not be enough carbon for the de-nitrifying bacteria to function if the BOD loading is unusually low; in this less likely scenario, additional carbon would need to be introduced to the influent stream.

Finally, as you stroll through the facility, you observe unattractive little clumps of floating muck gathering on the clarifier’s surface. Knowing that your sludge blanket at the bottom of the clarifier is becoming anoxic and nitrifying, causing sludge to float as a result of the production of nitrogen bubbles, you immediately recall this helpful article discussing this exact situation. Letting instinct take over, you spring into action by increasing the recycle rate, lowering the sludge inventory time in the clarifier, and preventing this unwanted denitrification.
In conclusion, a wastewater treatment facility improves the nitrification and denitrification environment to help nitrogen move through the nitrogen cycle. Wastewater treatment facilities thereby reduce the negative effects of human activity on our environment and freshwater resources.
Nitrogen Removal from drinking water
Given that nutrients are necessary for life, nitrogen found in wastewater from the preparation of meat is referred to as a nutrient. They mostly came from proteins dissolved in effluent from stockyards, beef tissue, blood, and paunch fluids. Australia has strict regulations regarding nitrogen.

These parameters specify the values that the wastewater treatment facility must consistently meet and, to some extent, the best treatment methods. In Australia, there are several different regulatory restrictions on river discharge. These restrictions are typically negotiated on a site-specific basis, taking into account the limiting nutrient of the river system and the watershed.
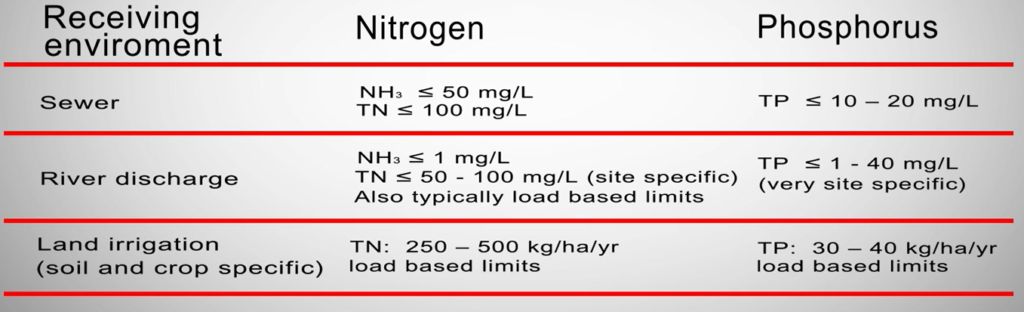
A variety of procedures exist for decreasing nitrogen concentrations in meat processing effluent, however in many areas of Australia, direct river discharge is not an option. Some limitations are concentration-based, while others may be defined as load base limits, notably for land irrigation.

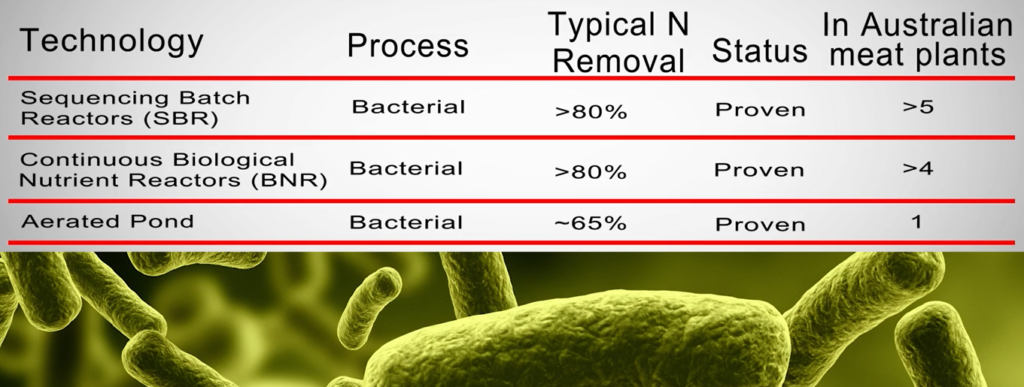
The first three technologies in the table below use biological bacterial nitrogen removal techniques involving activated sludge.
Biological nitrogen removal technology
For river discharge and field irrigation, biological nitrogen removal technology is recommended, or nitrogen limitations are enforced. Many different types of reactors can use biological nitrogen removal, including.
- biological nutrition reactors operating continuously.
- synchronisation, batch reactors.
- Aquatic ponds
A less demanding variation of continuous BNR systems.
In this section of the article, the first two technologies will be highlighted. In addition to struvite and anaerobic ammonium removal technology, DAF technology is frequently employed for sewage discharge.
Nitrogen Removal using activated sludge treatment
Nitrogen removal is accomplished via the biological nutrient removal method of activated sludge in two steps. Ammonia oxidising bacteria convert ammonia-rich water from upstream cows or anaerobic lagoons to nitrite in the first step of the nitrification process, which is a multi-step reaction.
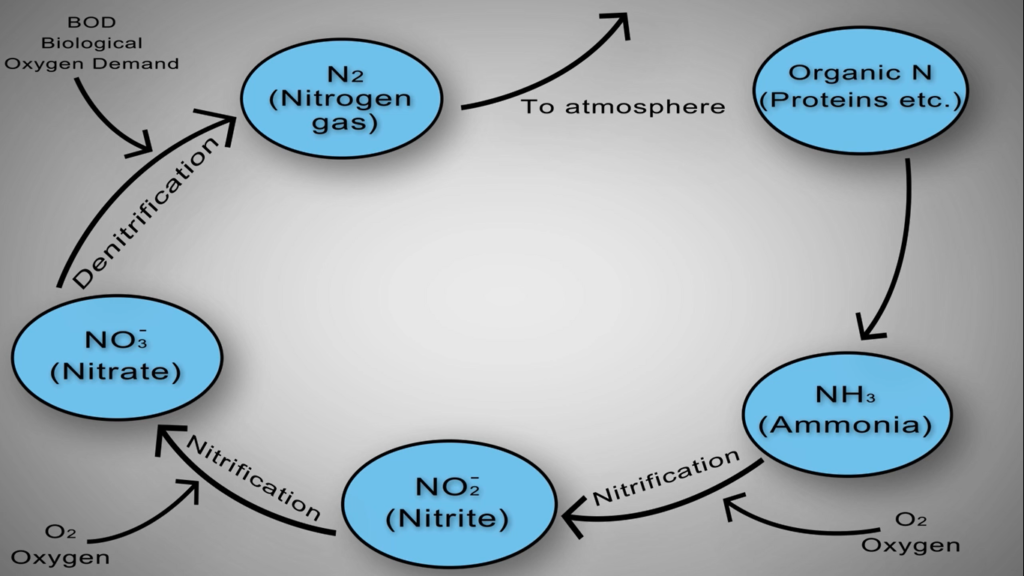
By nitrite oxidising bacteria, nitrite is further oxidised to nitrite. Although nitrification only modifies the dissolved nitrogen in the wastewater, it is a crucial first step in the removal process. The nitrification process must take place in an aerated zone with access to oxygen and the lowest feasible COD and BOD levels.

The second step is the de nitrification process, in which microorganisms convert nitrate to dinitrogen gas. The nitrogen is removed from the wastewater by the nitrogen gas that escapes into the atmosphere. In the absence of oxygen, the de nitrification process necessitates large concentrations of biodegradable COD.

Each phase requires a completely different atmosphere. These two distinct settings are made available by the two technologies in various ways. Nitrification and denitrification are carried out in various areas of a continuous biological nutrition reactor.
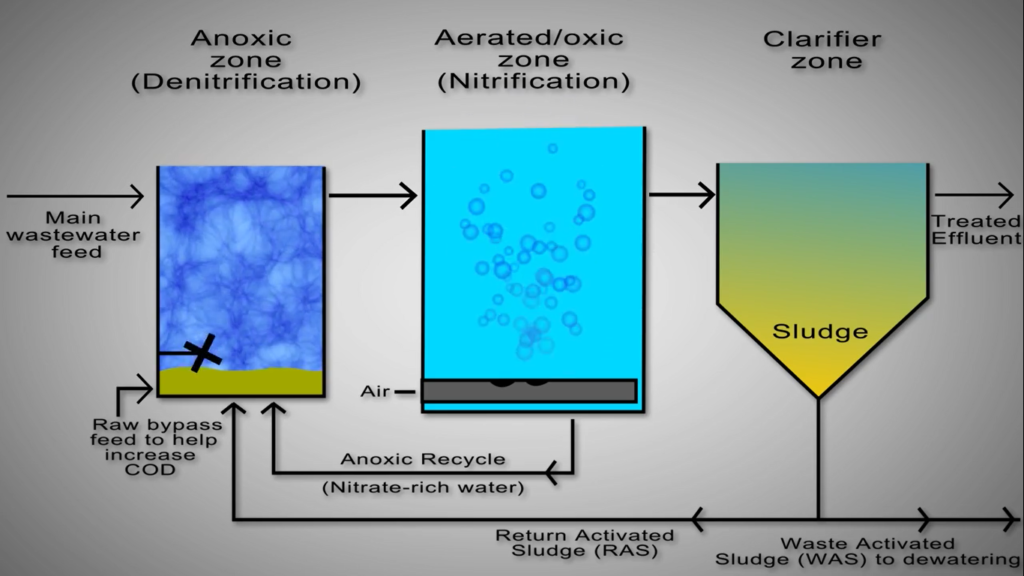
As an illustration, consider the Bio Lab method, where despite the lack of walls between the nitrification and denitrification zones, there are specific sections designated for each reaction. To finish the reaction, this calls for steady flow between the zones.
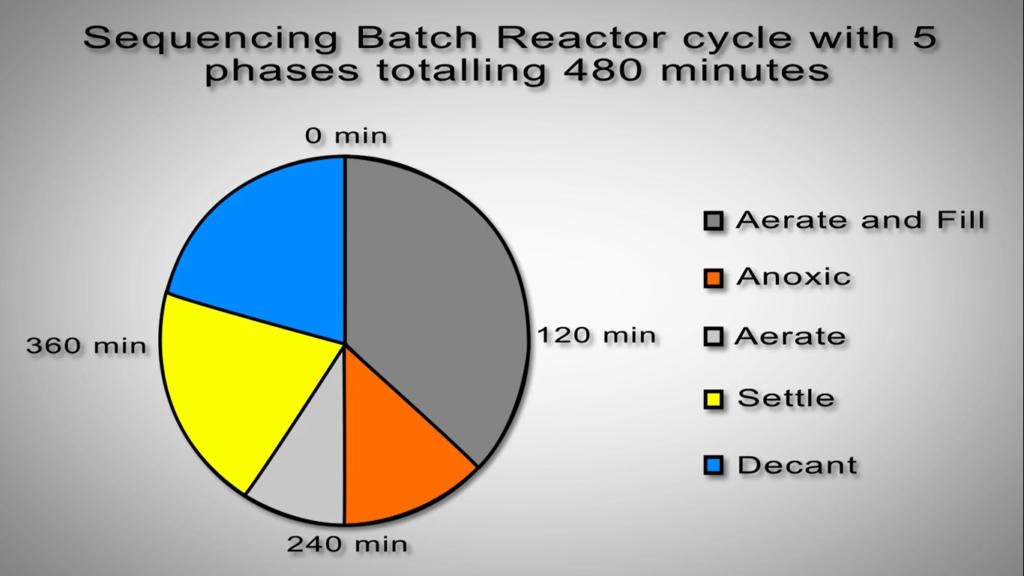
Sequencing Batch Reactors
Nitrification and de nitrification take place in the same reactor space but at different points in the cycle in sequencing batch reactors. As an illustration, the aeration system starts up during the nitrification phase of the cycle and shuts down during the de nitrification phase. This eliminates the need to pump vast amounts of liquid or to use separate devices like clarifiers. The relevant bacteria will carry out the processes necessary to lower nitrogen levels provided the right environment is there.
An anoxic de nitrification basin ozone, based on ozone, continuously receives numerous streams, including the main feed of ammonia-rich wastewater from the upstream cal or anaerobic pond, is one of the key components of a modern continuous biological nutrient reactor activated sludge systems. The majority of the additional nitrogen load to the system is present in this huge volume stream.

Unfortunately, this stream’s COD level is frequently insufficient to give the de-nitrifying bacteria all the COD they require. Since adding more COD is expensive, most systems send some of the raw, primary processed wastewater to the anoxic basin instead to provide the necessary carbon. Since this stream avoids the upstream Cal, this stream is frequently referred to as the raw bypass.
The return activated sludge is often pumped out of the bottom of the clarifier, and for certain systems, this can be as much as 25% of the entire wastewater flow. The nitrifying bacteria need nitrate, which is not present in the raw wastewater feed and must be provided through recycling from the downstream aeration basin, to function, ensuring quick rates of treatment. The upstream aerated zone in the biotech process supplies the water that is rich in nitrates to the downstream anoxic zone.

There is not a separate stream for recycling. Typically, the basin is mixed with submerged mixes to reduce the amount of oxygen present. De-nitrifying microorganisms in the anoxic basin catalyse the transformation of nitrate into nitrogen gas while also consuming COD. Wastewater continuously flows out of the anoxic base and ozone into the aerated base in ozone, maintaining high dissolved oxygen concentrations. Nitrogen gas loss to the atmosphere eliminates nitrogen from the wastewater.
The alkalinity produced by both the upstream calcifying or anaerobic pond and de-nitrification balances the acid emitted by the nitrification process. Continuously flowing into the clarifier are the components of the aeration basin. If the treatment effluent overflows the clarifier, which usually occurs through some sort of waste system, and has low levels of total suspended solids (typically less than 20 to 50 milligrams per litre), the bacterial flock settles out of the water column in the steel environment.
The settled sludge is pushed out of the bottom of the clarifier and disperses into the recycling and dewatering plants for disposal sludge.

A belt filter press or a decanter centrifuge are frequently used as the dewatering device in wastewater treatment facilities for red meat processing. An appropriate polymer is injected into the sludge stream to encourage a substantial, stable flow for dewatering. The combination is pumped to the dewatering mechanism, where it is greatly reduced in water content and produces a dewatered and sludge cake.

The filtrate should ideally resemble wet cardboard, and it should be returned to the system’s head while the sludge cake is disposed of at a suitable disposal site like a landfill or composting facility.
Systems that use activated sludge in a continuous biological nutrition reactor have relatively low operating costs, low maintenance needs, and can produce high-quality effluent. However, they require expert operators and are inflexible to changes in wastewater volume or quality. They also have significant operating expenses, produce a lot of sludge, and need a lot of wastewaters to be circulated.
Sequencing Batch Reactors Biological Nitrogen Removal
Sequencing Batch Reactors operate the anoxic, aerated, and settling processes in various specialised tanks, each with its own equipment, as opposed to performing the activated sludge process in batches. The Sequencing Batch Reactors perform each stage in a single tank or pond but at various times.

The reactor is different in a sequence batch reactor because a time-based control method is used, but the biological processes are the same. This method is based on a cycle that has several phases. By regulating the admission, exit, and use of equipment, each of these steps creates the environment necessary for the process.
Automation is used, and PLC control is used. The cycle clock resets to zero after a Sequencing Batch Reactor cycle is finished, and the Reactor then repeats the cycle with a fresh batch of wastewater. A residential dishwasher that runs with one batch of dirty plates every cycle and features time-separated rinse, wash, and dry phases is a better comparison to the Sequencing Batch Reactors. Waste activated sludge still needs to be eliminated.

Sludge is typically removed from Waterman Engineers Australia during the aerate and fill phase using a pump, just like in continuous biological nutrient reactor plants. The operating flexibility of sequencing batch reactors is quite high in response to variations in effluent or quality. Due to its single-tank operation, the Sequencing Batch Reactor does not require an expensive clarifier or sludge recycling streams, which typically require extensive pump and pipe circuits and raise capital expenses.
Although they have a high level of automation, a reduced footprint, and good effluent quality that can meet present and predicted future discharge regulations, they do need a decanter mechanism that runs during the decant cycle to remove clarified water. However, compared to continuous biological nutrient removal facilities, they do demand a higher level of control and call for informed and skilled operators.
Additionally, they need an upstream storage facility to keep wastewater during periods when it would not be able to enter the sequencing batch reactor. Twin Sequencing Batch Reactors can also be utilised in simultaneously, as might an upstream Cal or anaerobic pond.
Legislative and regulatory requirement
The facility’s environmental protection licencing permission or licence, which is given by the state government, will contain specific regulatory criteria regarding nutrient removal. Typically, the main emphasis is on reducing the possibility of odour, particularly from biological nutrient removal systems, and on the proper off-site transport and disposal of the dewatered waste solids.

Operator responsibilities
biological nutrient removal methods for activated sludge that demand constant operator attention However, at the right points in the cycle, daily operator attention was also needed for sequencing batch reactors. Activated sludge biological nutrient removal systems are particularly responsive due to their relatively short hydraulic retention durations and their high bacterial densities, therefore most operator time is spent observing performance.

Because treated effluent must adhere to strict quality standards in order to be discharged into rivers or used for high-quality reuse, it is crucial that their performance is constantly assessed. To remove and dewater the sludge from the system, operators of the dewatering devices must work multiple times a week. The watering behaviour is complex and influenced by a variety of variables, including pH, weather, sludge settling behaviour, and many others.
To ensure the proper dewatering conditions and polymer addition amounts are achieved each time the device is used, it takes some time. A major issue exists when sludge does not settle, and immediate action is required. The total suspended particles in the final effluent often increase as a result. At least once each day, sample and test the sludge from the aeration basin’s ability to settle. If your ability significantly changes, notify your supervisor right away.

Regular inspections should be performed on pumps, inlet and output pipelines, and aerators. Examine the basins for accumulating amounts of scum, foam, crust, or mousse that resembles fat or pavlova mixture. This can indicate bacterial bulking. Inform the supervisor so they can decide how to proceed and make sure any necessary chemicals are on hand. It is crucial that any adjustments or system operations are made thoughtfully and in accordance with the operational guidelines specified by the designers.
Copper Removal Using Ion Exchange Resin
Copper is frequently present in industrial wastewater from processes such nonferrous smelting, electroplating, chemical printing, and dyeing. Cu2+ can be successfully removed from wastewater using ion exchange resin to produce high purification, which is helpful for resource regeneration and environmental protection.

Waterman Engineers Australia have developed ion exchange resins specifically designed to remove copper from wastewater. This process is currently being used in Australia. If you are interested contact us.
Ion exchange resin treatment of copper containing wastewater. The ion exchange resin is an organic polymer compound having a main body lattice structure. The number and type of reactive groups determine the total exchange capacity and selectivity of the resin, and the exchange capacity is the number of exchangeable ions in the resin.
- It has an index indicating the exchange capacity of the resin, it can be divided into
- Full exchange capacity meaning that all active groups and the exchange resin regenerate the total amount of exchangeable ions
Working exchange capacity refers to the resin in the exchange process the actual the total amount of exchangeable ions that act as an exchange, it is closely related to the actual operating conditions.
The type of impurities contained in the raw water, the concentration, the thickness of the exchange resin layer, the influent pH, temperature flow rate, resin regeneration degree and other operating conditions.
Effective ion exchange resin on copper containing wastewater
- Quantitative reaction with the same mass as other chemical reactions
- It is a reversible reaction that follows the law of mass action
- The exchanger is selective and the exchanged ions on the exchange are first exchanged with the exchanged ions
- The ion exchanger is applied to wastewater treatment and the material can be recovered. For example, the copper containing wastewater is first exchanged with each type of cat ion resin and the cat eye in the wastewater is exchanged.
Iron and Manganese Treatment
Iron and manganese are removed from water by the principles of oxidation and precipitation along with filtration. So, I’m going to show you what happens to oxidise and then precipitate the iron and manganese out and then filter it out.
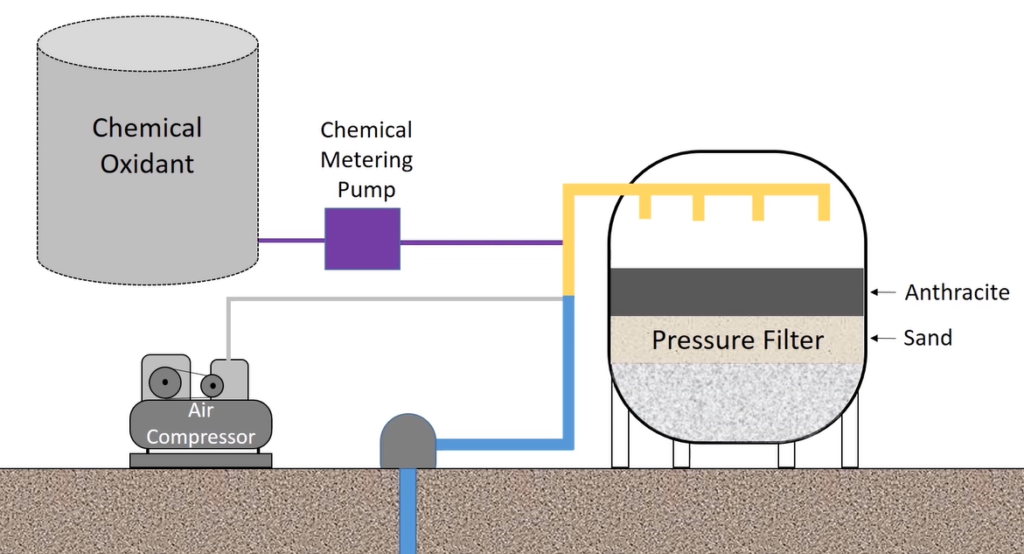
So, I’m going to look within these red lines here. What we have here is a well, that’s pulling water out of the ground, and it is high in iron and manganese. And the iron manganese at this point is in solution. So, it’s soluble, it has not precipitated out yet. But if we don’t deal with these high concentrations of iron and manganese, when they do become oxidised, such as when they come out in their oxidised by the air in a glass in the customer’s home, or in a sink or in the bathtub, the exposure of the iron and manganese in the water to the oxygen in the air will cause it to oxidise and then come out of solution and change colours.
So, what we want to do while it’s still in solution, what we do is we oxidise it. And there’s a couple of ways we can do that, we can use an air compressor and inject air which contains oxygen into the flow stream, which would cause oxidation. So, we’re on purpose trying to get the iron and manganese that’s in solution to precipitate out of solution so we can filter it out.
One method is by using air or some form of aeration. Here we have an air compressor. But it could be some other form of aeration, where another type of aerator is used to oxidise iron more so than manganese. Manganese typically requires chemical oxidation.
We have our chemical oxidant. It could be chlorine, it could be potassium per magnate, it could be ozone. But at any rate, some chemical is used to oxidise the iron and the manganese that’s in solution. we’ll just pretend that it’s potassium per magnate, since our feed line in our feed pump is purple potassium per magnate comes in purple crystals and it turns the water kind of a pinkish colour pinkish purplish when it’s dissolved in the water.
But just like the air, that chemical can be injected into the flow stream, and it causes oxidation to occur. last portion which is the filter. We have a pressure filter, where you can see that the water coming out of the well was blue or clear. We added the oxidant either the aeration or the chemical oxidant and it caused the iron to turn this yellow rust colour What happens now is the iron and the manganese that’s oxidised will precipitate out and it will get stuck in the anthracite and sand filter and then the water passing through the filter no longer has that colour and it turns back to its normal clear colour again. So, we started clear because the iron and manganese were dissolved in solution. We then oxidised the iron and manganese, so it precipitated out and the precipitates stayed in the filter. And that clear clean water then goes on to the distribution system to the customer’s home.