Multi-Effect Distillation
It is method of component separation from liquid mixture, depending on the differences in boiling points of the individual components and the distributions of the components between a liquid and gas phase in the mixture.
Overview
The liquid mixture has different boiling point characteristics depending on the concentrations of the components in it. The vapor pressure characteristics of slurries determine the distillation process. The vapor pressure is produced by supplying heat as a separating agent. The next phases differ from the original by their heat content. During recent times, distillation was by far the most extensively used method for separating liquid mixtures of chemical components.
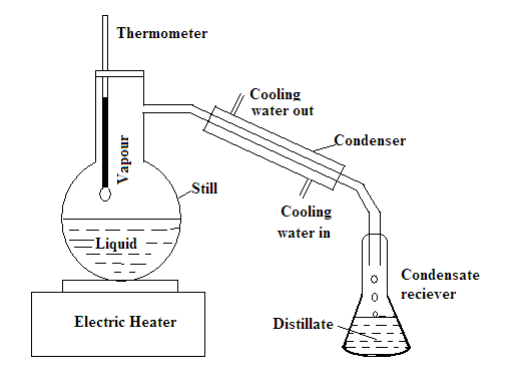
This process needs the use of a still in which a liquid is heated, a condenser to cool the vapour, and a receiver to collect the distillate.
Applications
Distillation process finds its application in manifold:
- Purifying water
- Producing Gasoline
- Recycling oils
- Processing of formaldehyde and phenol and the desalination of seawater.
Advantages
- Separate liquids from non-volatile solids.
- Alcoholic liquors separation from fermented materials.
- Separation of liquids which have different boiling points.
- Gasoline, kerosene, and lubricating oil separation from crude oil.
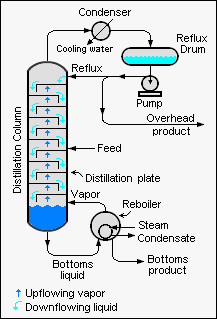
Fractional distillation
It is a type of distillation used for separating liquids whose boiling points lie close to one another.
Overview
In this operation the vapours from a distillation are continually condensed and re-vaporized in an insulated vertical column. Important connections are the still heads, fractionating columns, and condensers that allow the return of some of the condensed vapour toward the still.
Generally, the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under the pressure of one atmosphere. If the difference in boiling points is above 25 °C, a simple distillation is usually used.
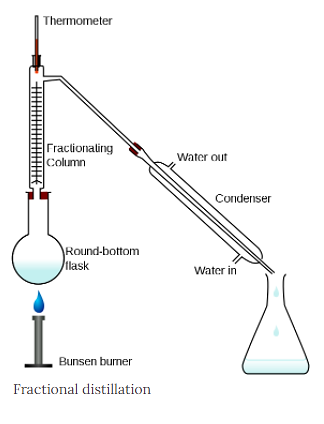
The fractional distillation apparatus is set up with the heat source at the bottom of the still pot. As the distance from the stillpot rises, a temperature gradient is generated in the column; it is coolest at the top and hottest at the bottom.
Application
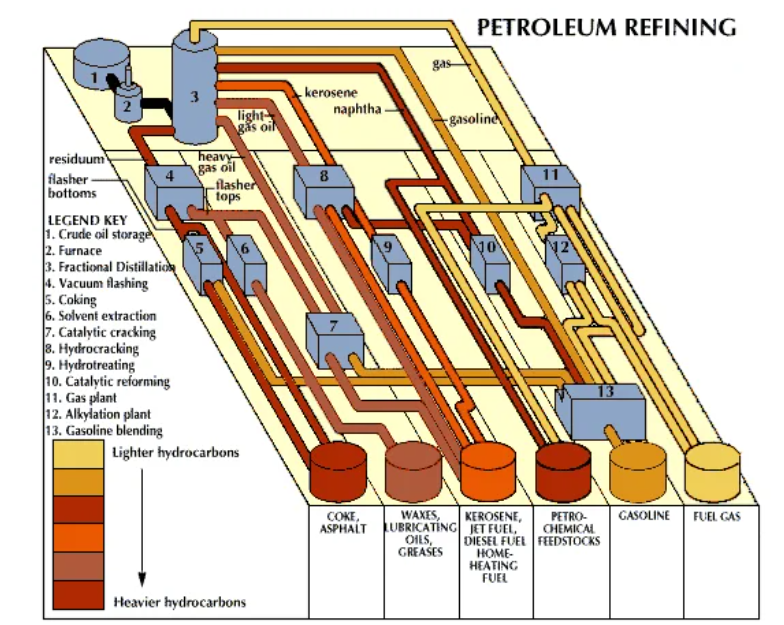
Petroleum Refining
Advantages
- It is able to handle any waste streams
- works with any size of industry
- More efficient than simple distillation equipment
Steam distillation
It is an alternative method of getting distillation at temperatures lower than the normal boiling point.
Overview
The procedure is to pass steam into the liquid in the still to supply heat that causes evaporation of the liquid.
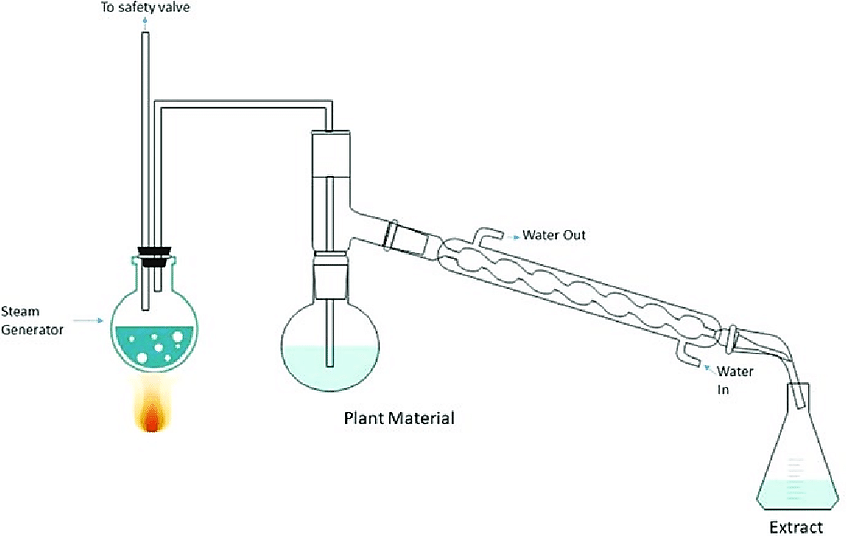
It is applicable when the material to be distilled is immiscible and chemically unreactive with water.
Application
It is widely used in the manufacturing of essential oils, for instance perfumes.
Advantages
- Organic solvent-free products
- No need for subsequent separation steps
- Large processing capacity in the industrial scale
- Inexpensive equipment
Vacuum distillation
The reduced-pressure process difference uses a vacuum pump to generate a very high vacuum. This method is called vacuum distillation.
Overview
This type of distillation is used when dealing with substances that usually boil at inconveniently high temperatures or that decompose when boiling under atmospheric pressure.
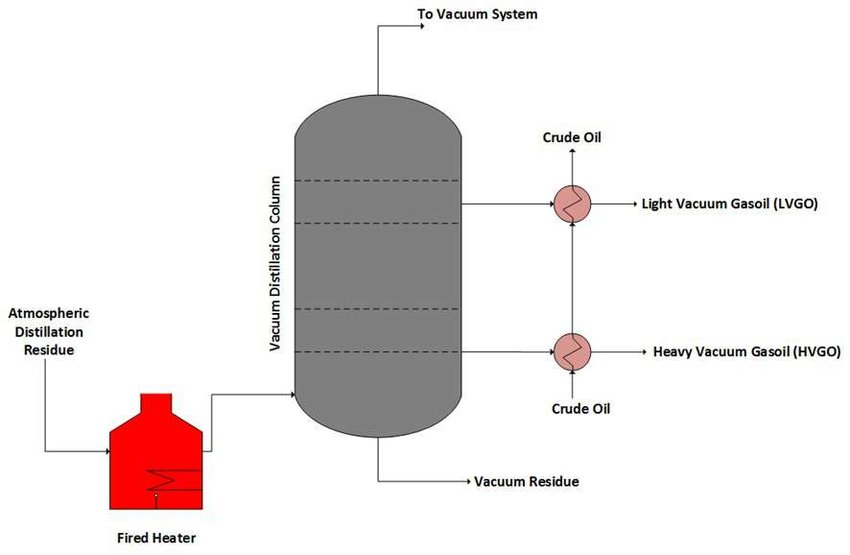
It works by lowering the pressure in the column above the solvent to less than the vapour pressure of the mixture, creating a vacuum, and causing the elements with lower vapour pressures to evaporate off.
Application
- Production of food and beverage to extract plant essences
- Separation of long-chain hydrocarbons in oil refineries
Advantages
- Faster processing time
- Effective distillation
- Efficient processing of higher boiling point solvents without igniting them or causing thermal breakdown.
Air-sensitive vacuum distillation
The type of distillation used for compounds having high boiling points or air sensitive compounds.
Overview
Some compounds have high boiling points as well as, air sensitive. A simple vacuum distillation system can be used, but the vacuum is replaced with an inert gas after the distillation is complete. But this is a less satisfactory system if one desires to collect fractions under a reduced pressure. For this a “cow” or “pig” adaptor can be used at the end of the condenser, or for better results or for very air sensitive compounds a Perkin triangle apparatus can be used.
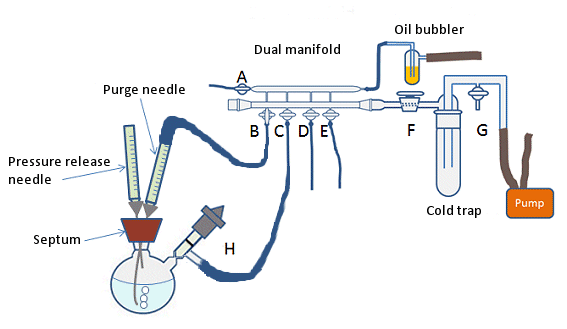
Application
It is useful for compounds which boil beyond their decomposition temperature at atmospheric pressure and therefore be decomposed by any attempt to boil them under atmospheric pressure.
Short path distillation
It is a distillation technique in which the distillate travels a short distance, often only a few centimetres, and is normally done at reduced pressure.
Overview
It is used for compounds which are unsettled at high temperatures. A short path makes sures that little compound is lost on the sides of the apparatus. The Kugelrohr apparatus is a kind of short path distillation method which often contains multiple chambers to collect distillate fractions.
Short path vacuum distillation apparatus with vertical condenser (cold finger), to reduce the distillation path:
- Still pot with stirrer bar/anti-bumping granules
- Cold finger – bent to direct condensate
- Cooling water out
- cooling water in
- Vacuum/gas inlet
- Distillate flask/distillate.
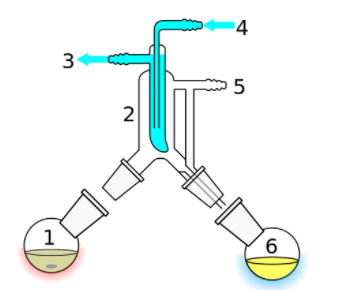
This technique is generally used for compounds which are unstable at high temperatures or to purify small amounts of compound.
Application
Food and pharmaceutical industries
Advantage
The heating temperature can be thoughtfully lower (at reduced pressure) than the boiling point of the liquid at standard pressure, and the distillate only has to travel a short distance before condensing.
Zone distillation
It is a distillation process in a long equipment with partial melting of refined matter in the moving liquid zone and condensation of vapour in the solid phase at condensate pulling in the cold region.
Overview
The process works in theory. When the zone heater is moving from the top to the bottom of the container then solid condensate with irregular impurity distribution is forming. Then most pure parts of the condensate may be extracted as a product.
The process may be repeated many times by moving the received condensate to the bottom part of the container in place of refined matter. The irregular impurity spread in the condensate (that is efficiency of purification) increases with the number of iterations. Zone distillation is the distillation counterpart of zone recrystallization. Impurity distribution in the condensate is explained by known equations of zone recrystallization.
Application
products like alcoholic beverages, juices, and many other things are purified using zone distillation method.
Advantages
In many industrial zones, different products are refined by using the process of chemical synthesis through zone distillation.
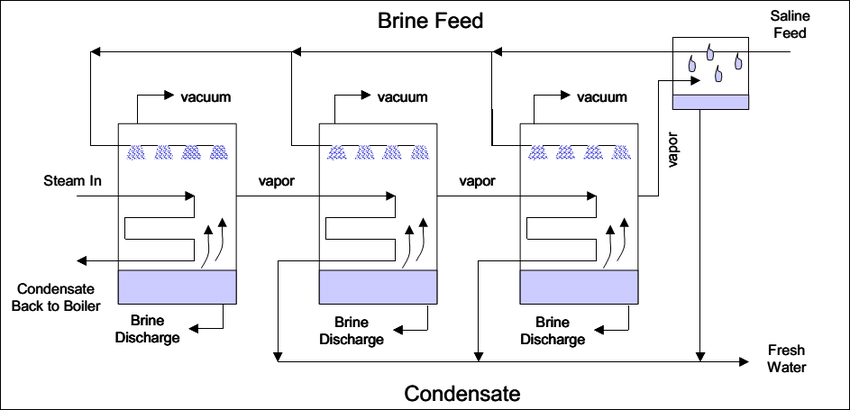
Multi Effect Distillation
Multiple-effect distillation (MED) is a process that includes multiple effects. The feed water is agitated in tubes with steam in each effect, generally by spraying saline water on them. Portion of the water evaporates, and the steam enters the tubes of the next effect.
Overview
Multiple effect distillation (MED) at present, the preferred technology for the construction of new plants based on thermal processes in the growing desalination market. It is, in fact, presents a number of advantages with respect to the more traditional multistage flash technology, among all the lower energy consumption achievable in MED plants.
MED Advantages:
- Very low electrical consumption (<1.0 kWh m−3)
- Works at low temperature (<70 °C) and at low concentration (<1.5)
- No need of complex pre-treatment of seawater and are tolerant to variations of seawater conditions
- Highly reliable, simple to operate
- Reduces civil works cost
- Simple to install
- Low maintenance cost
Application as an Effluent management:
Most of the existing desalination processes, e.g. reverse osmosis (RO) and multi-stage flash desalination (MSF), can only separate a certain portion of freshwater (~50%) from seawater. The resting stream, which is named brine, has a higher salinity than normal seawater. Existing brine treatment methods usually discharge brine directly into the ocean or surface water. They have many harmful effects on the environment.
One sustainable option for effluent (brine) treatment is zero liquid discharge (ZLD) systems that completely convert seawater into fresh-water and salts.
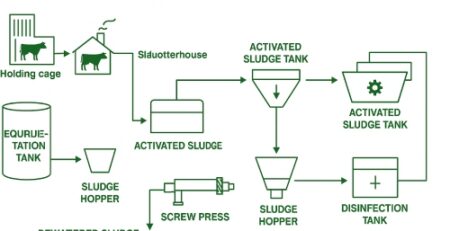
The ZLD system consists of a MED system and an evaporative crystallizer. The MED is arranged in a forward-feed manner such that maximum concentration occurs in the last effect that has the lowest temperature and the risks of scaling and fouling are reduced. The
evaporative crystallizer consists of a heater, an evaporation/crystallisation chamber, a salt/brine separation device, and a condenser. Brine from a regular desalination plant is firstly concentrated in the MED system, and then the concentrated brine is lead to the crystallizer for complete separation of salt and water as in the given arrangement below:
Environmental Benefits:
Firstly, MED is used for brine concentration, having better stability and higher energy efficiency than MD systems. Secondly, since the evaporative crystallizer can further concentrate the brine, the effluent left the previous process does not need to be supersaturated, and the chances of scaling and fouling in the brine concentrator (MED) can be substantially reduced. Thirdly, both MED and evaporative crystallizer can handle large brine flowrates, making the system efficient than those based on MD and solar ponds. Finally, the system let the implementation of different low-grade heat sources, e.g. solar thermal energy and industrial waste heat, making the process more sustainable and environmentally friendly.