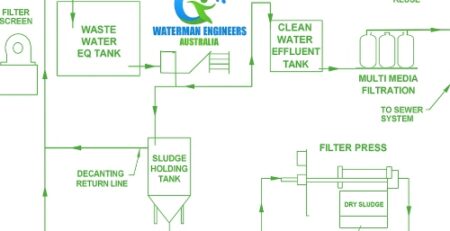
Waste Water Recovery from Tailings
If you are looking to recover water from tailings, there are several steps you can follow to make sure you do it safely. You have to learn what is involved, how to clean the water, and how it will impact aquatic and other organisms.
What are Tailings and How to Recover Waste Water from them?
The recovery of wastewater from tailings requires a variety of approaches. These include in-place stabilization, Phyto stabilization, and capping. In order to determine which approach works best, an evaluation of four treatments was conducted.
Tailings are waste generated during the processing of metal ore. They are often deposited into a pond. However, these ponds are susceptible to environmental issues due to the poor management of water. As a result, effective treatment is important for these facilities.
Tailings are often classified as slurry or sludge. The latter refers to a mixture of fine gangue particles and process-affected water. Tailings sludge can contain dissolved metals that can contaminate the groundwater.
Reprocessing of these materials is a viable alternative for tailings recovery. This significant study evaluated the effect of reprocessing on the concentrations of heavy metals in slurry and sludge. For the sludge, a high-grade sulphide concentrate was generated. Compared to the bulk tailings, this sulphide concentrate contained more than four times the copper and more than two times the sulphide sulphur.
Lime addition is another viable treatment for recovering water from tailings. It has been found that lime-amended tailings are more suitable for vegetative growth than unamended tailings.
Plant growth was examined in each of the four treatment treatments. Two plant species were used for this experiment. Seeds of Kentucky bluegrass and a grass species suited for the sand mining industry were planted.
After 120 days of planting, the above-ground biomass was collected. The topsoil control showed no significant differences in vegetation establishment compared to the other treatments.
How to Achieve Neutralization of Tailings Water?
Mining tailings are waste materials from extracting processes. Tailings contain dissolved metals and organic species. These contaminants must be treated before disposal.
Mine tailings represent a major environmental liability. Several reliable studies have been conducted on their potential and limitations.
Laboratory experiments assessed the effectiveness of three possible mechanisms. The effects of pH, reaction time, and dosage of NaClO were examined. Moreover, the effect of colloidal particle adsorption was tested.
A slurry is a mixture of fine gangue particles. During the treatment process, the slurry is circulated back to the process to increase reaction kinetics. CYH is a cationic coagulant which adsorbed through electrostatic force.
In order to achieve a proper neutralization of tailings water, an oxidation-sedimentation treatment was performed. This process was carried out at an iron mine concentrator in Shanxi province, China.
After standing for several hours, the supernatant was separated. A KI-starch paper was used to record the reaction progress. It was discovered that the oxidation-sedimentation process improved the overall waste water quality.
In addition to the aforementioned, the effects of PDADMAC on flotation iron recovery were also examined. Similarly, the impact of redox conditions on metalloid solubility was examined.
CYH has a high binding force and forms large flocs. Consequently, it is considered as an efficient coagulant.
What is Chitosan in Wastewater Treatment?
Chitosan is a versatile polymer with a cationic charge, which is used as a sorbent in wastewater treatment. It has a high reactivity and is able to adsorb pollutants from aqueous solutions. It is an effective sorbent and coagulant for a variety of industrial wastewaters and can be chemically modified to increase its adsorption capacity.
Chitosan is a unique alternative adsorbent for toxic metals. It can be used to remove a wide range of heavy metals from aqueous solutions.
Many research studies have shown the biosorbent properties of chitosan. The use of chitosan as an adsorbent is especially beneficial in specialized applications such as the recovery of waste water from tailings.
In one reliable study, adsorption of Pd(II) was investigated on S-modified chitosan adsorbents. These adsorbents showed the highest selectivity for the precious metal. However, a decrease in the uptake efficiency was observed when the presence of chloride ions or nitrate ions was present.
Moreover, the presence of sulphur ions also decreased the uptake efficiency. This was because these ions tend to bind to the amine groups of chitosan and have greater binding affinity.
Several biomaterials have been studied as potential coagulants and flocculants. Some of them are chitosan and its composites. They can also be biodegradable, are low in toxicity, and are suitable for a variety of applications.
Chitosan-zeolite composites are a promising adsorbent material for organic impurities from industrial wastewater. Because of their large surface area and mechanical strength, zeolite-chitosan can be used for large-scale wastewater treatment. Compared to unmodified chitosan, the sorption capacity of the zeolite-chitosan composites increases.
Another study demonstrated the sorption and desorption of Au(III) from aqueous solutions containing chitosan. The removal efficiency of chitosan is highly dependent on the pH of the solution and the amount of competing ions. For best results, chitosan should be treated at a pH of 3.
Surface Stacking Technique for Waste Water Recovery from Tailings
The surface stacking technique for waste water recovery from tailings is an effective solution for wet or dry climates. It provides significant advantages over ponding and also lowers the risk of containment breaches.
This method of disposal is not for every mine, though. However, it is a valuable tool for smaller operations and areas with restricted access.
A major advantage of the surface stacking technique is its ability to recover more than 90 percent of the water. That is a huge water savings. In arid areas, the savings can far outweigh the cost of the solution.
The process of forming the sludge stack requires careful analysis of the site’s geology and geotechnical conditions. It also involves detailed tailings geotechnical testing. Changes to the outlet structure or the drying pan can be made to direct the sludge flow.
Surface stacking for tailings disposal can reduce the capital and operating costs of a tailing’s storage facility. It also minimizes the complexity of permitting.
Dry stacked filtered tailings are often used as a water supply reservoir for plants. They are usually transported by truck. Since they have less moisture content than ponded tailings, they can be used in self-supporting structural situations.
In a cold climate, heated bed liners are essential. Thin layers of applied sludge consolidate more than thick layers. Combined with proper compaction, this technique can prevent erosion.
Impacts of Waste Rock on Aquatic and Other Organisms
Mining waste is a hazardous material that has a large impact on aquatic and other organisms. The effects of these contaminants can be categorized according to their nature and origin. In aquatic environments, adsorption and precipitation are the main mechanisms to remove highly toxic elements.
The mobility of trace elements depends on the oxidation-reduction process, complexation, chelation, and sorption. These processes are influenced by the nature of the minerals and sediments.
The most important process, sorption, supports ions at the interface of the solid and aqueous phases. This is an important transport mechanism. It can be enhanced by heat, which is released during the oxidation process.
Sedimentation is another process that can remove heavy elements through adsorption or precipitation. In bed stream sediments, red colouring results from the abundant precipitation of Fe(III)-hydroxides.
Metals are also transported to the stormwater system via runoff. The concentrations of a specific element will depend on the depth of the ore deposits, its permeability, and the buffering capabilities of the host rock.
Mercury is also a major source of environmental pollution. This toxic element can be mixed with soil and waterways, as well as the aquifer. While it does not contaminate the environment directly, it is known to disrupt natural bacterial populations. Moreover, it may have a significant effect on nutrient absorption from soil to plants.
Depending on the source, mining waste can reach aquatic environments by runoff or acid mine drainage. The amount of heavy metals in wastewater varies from a few mg/kg to a few hundred grams.
Some minerals have high adsorption capacity for highly toxic elements. Pyrrhotite is a good example of this. Other gangue minerals include arsenopyrite (FeAsS), quartz, and calcite.
In Conclusion
Tailings recycling methods have many applications including mine recovery, stony desertification control, urban greening, and a range of other uses. They can also replace conventional mellow soil for plant growth.