Characteristics and Pollution Involved in Coal Mining
How is coal formed?
I have a piece of organic sedimentary rock which is coal. As you can see are getting all black and this is a property of good quality coal. Before we go into much detail, let me give you some context.

Coal, then, is formed by the collection and preservation of plant components, often in a swamp setting, which is a forest wetland. This type of coal is the result of millions of years of optimal geological conditions, specifically the right balance of plant matter, water, and heat pressure.
 Now, basically, there are five types of coal, and they are
Now, basically, there are five types of coal, and they are
- Peat,
- Lignite,
- Subbituminous,
- Bituminous
- Anthracite.
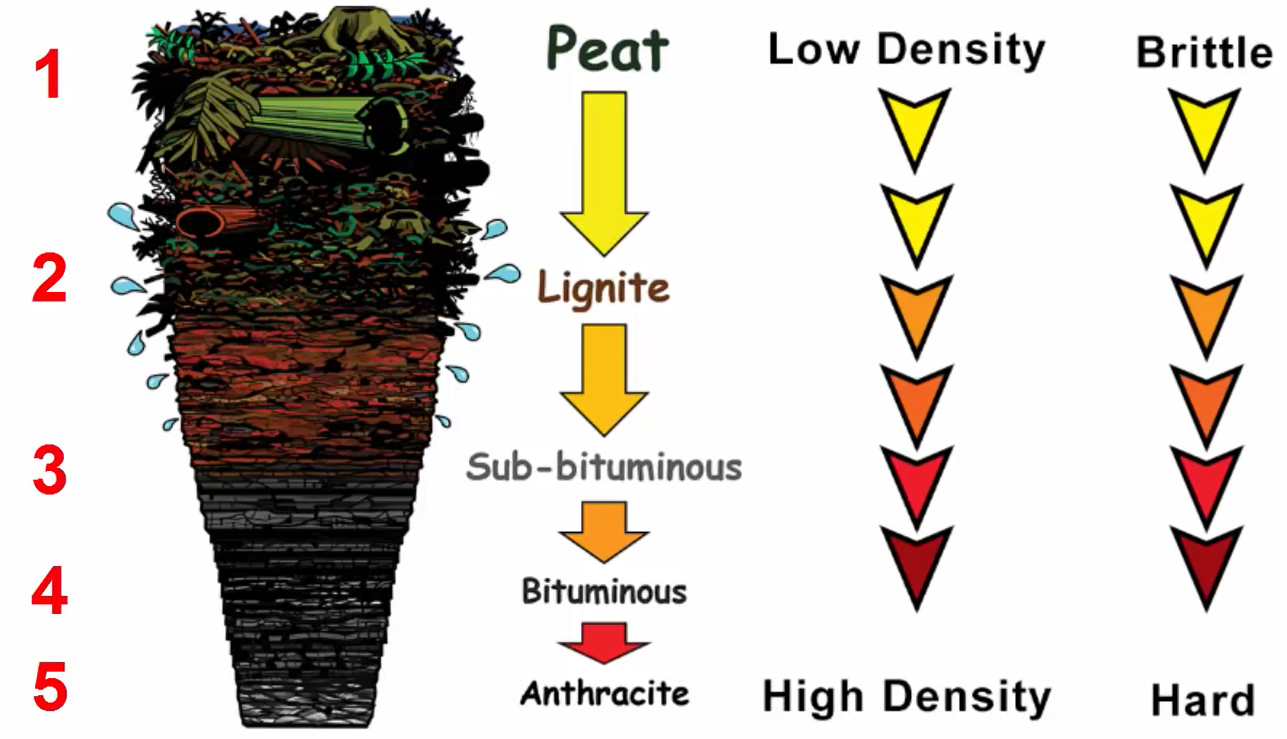
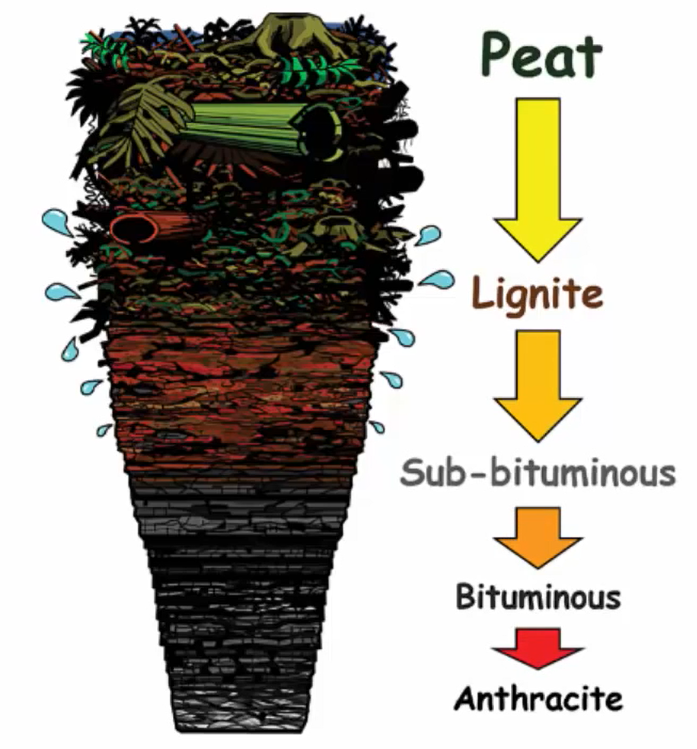 Let me show you all the stages through an illustration. The first stage consists of the beat, which contains decomposed plant debris like leaves, phone, branches, roots, etc. A nice example of beat would be this material, which is destined to become coal yet is still clearly identifiable for its intended use.
Let me show you all the stages through an illustration. The first stage consists of the beat, which contains decomposed plant debris like leaves, phone, branches, roots, etc. A nice example of beat would be this material, which is destined to become coal yet is still clearly identifiable for its intended use.
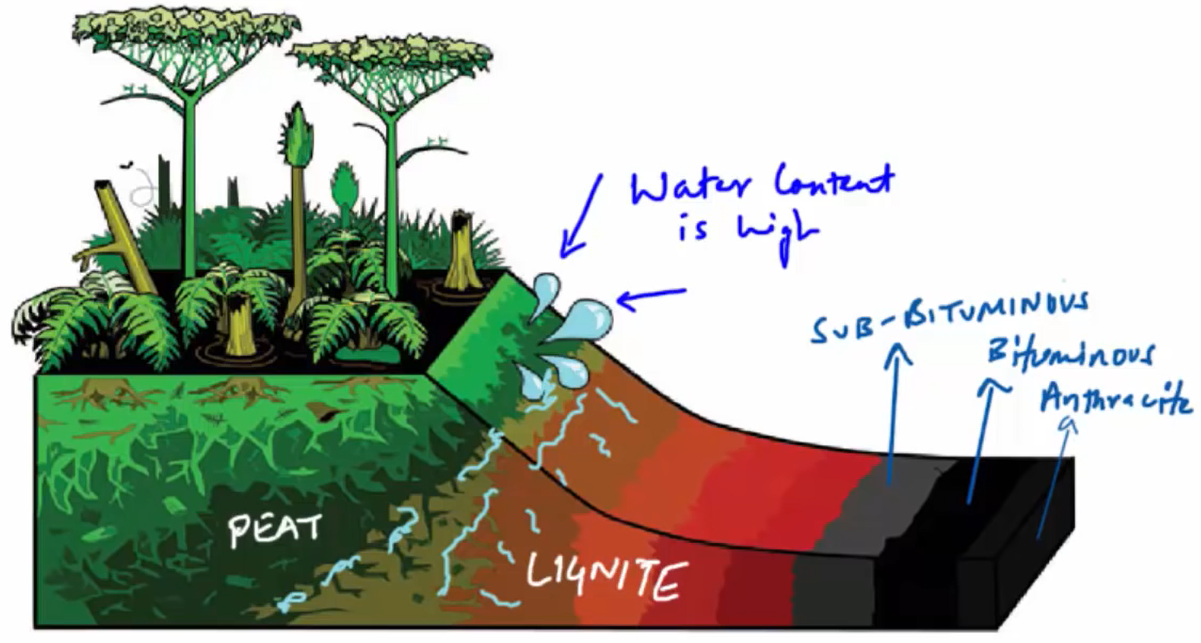 Lignite coal, sometimes known as brown coal, makes up the second stage. It’s easy to understand why. Due to its high moisture content, it degrades when exposed to air. This type of coal should not be kept in storage or transported across greater distances.
Lignite coal, sometimes known as brown coal, makes up the second stage. It’s easy to understand why. Due to its high moisture content, it degrades when exposed to air. This type of coal should not be kept in storage or transported across greater distances.
Coal from the third stage is subbituminous. The topmost layer of the primary bituminous coal is this. It also goes by the name Black lignite. The colour typically transitions from dark brown to black coal. Its characteristics ranged from lignite to bituminous coal.
This coal, which has a carbon content of 35 to 45 percent, is frequently utilised for industrial and steam power production. When sub-bituminous coal is exposed to air, it becomes unstable. It is thought to have developed at the same age as lignite and possesses characteristics that make it prone to disintegration.
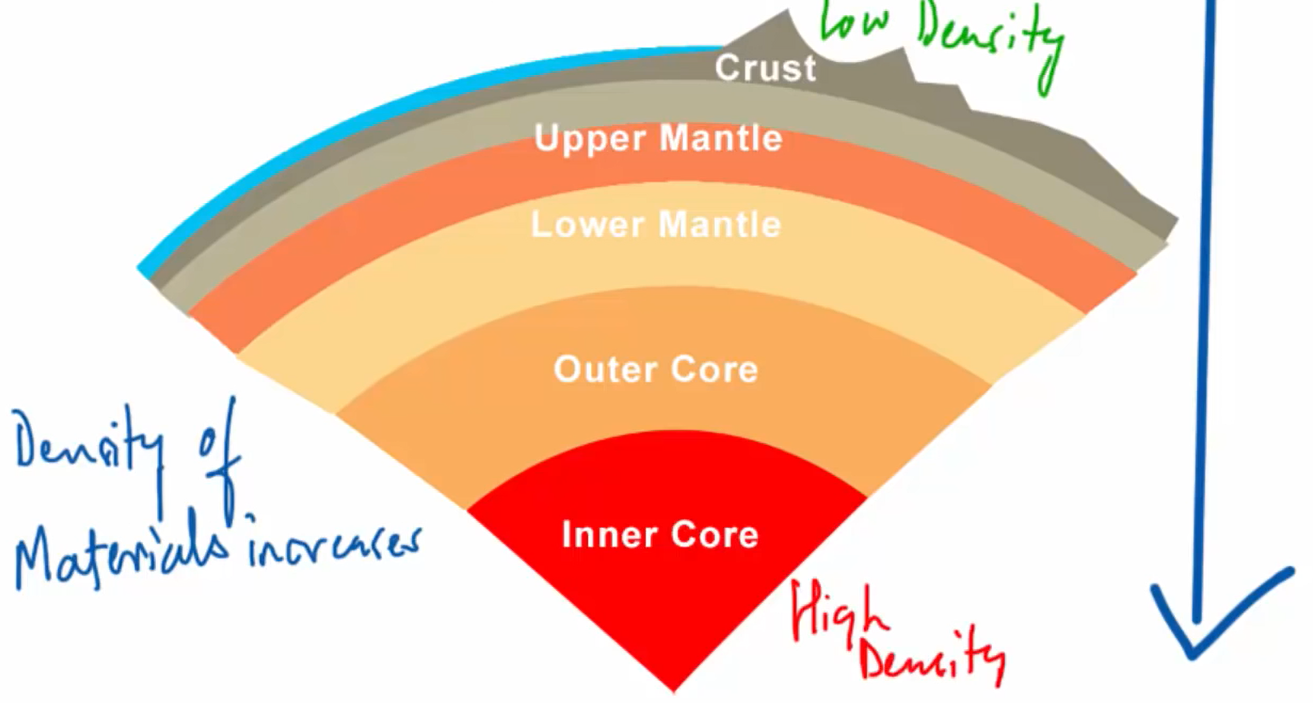
 The fourth stage of coal formation is now represented by bituminous coal, which is heavier than lignite or sub-bituminous coals and contains 60 to 80 percent carbon. According to what we’ve read, the density of materials increases as we move from the Earth’s crust to its core, which explains why this coal has a higher density than lignite or subbituminous coal. The most prevalent sort of coal is also this one.
The fourth stage of coal formation is now represented by bituminous coal, which is heavier than lignite or sub-bituminous coals and contains 60 to 80 percent carbon. According to what we’ve read, the density of materials increases as we move from the Earth’s crust to its core, which explains why this coal has a higher density than lignite or subbituminous coal. The most prevalent sort of coal is also this one.
 The anthracite coal is represented by the final and fifth stage. It is the best type of coal since it emits little pollution and has a high calorific value, which means it generates a lot of heat. With a carbon content of 75 to 95 percent, anthracite is extremely clean. It is extremely hot and has a very high density. This coal is somewhat glossy and has a high sub metallic shine.
The anthracite coal is represented by the final and fifth stage. It is the best type of coal since it emits little pollution and has a high calorific value, which means it generates a lot of heat. With a carbon content of 75 to 95 percent, anthracite is extremely clean. It is extremely hot and has a very high density. This coal is somewhat glossy and has a high sub metallic shine.
 So, this is all about the different types of goals that are available. Now I have two types of coal which I believe one is sub bituminous, and the other is bituminous coal. Let’s find out why I think so. The easy way to recognise this coal is by noticing its deep black colour. It means it has to occur between lignite, which is brown in colour and anthracite, which is shiny black in colour.
So, this is all about the different types of goals that are available. Now I have two types of coal which I believe one is sub bituminous, and the other is bituminous coal. Let’s find out why I think so. The easy way to recognise this coal is by noticing its deep black colour. It means it has to occur between lignite, which is brown in colour and anthracite, which is shiny black in colour.
 So, we have proven that these two are bituminous and sub bituminous coal. Now the question is which one is bituminous and subbituminous. Aside from the fact that lignite matures through time by being darker and harder, at which point it is categorised as sub-bituminous coal. More chemical and physical alterations take place as this process of burial and transformation occurs. After that, the coal is categorised as bituminous.
So, we have proven that these two are bituminous and sub bituminous coal. Now the question is which one is bituminous and subbituminous. Aside from the fact that lignite matures through time by being darker and harder, at which point it is categorised as sub-bituminous coal. More chemical and physical alterations take place as this process of burial and transformation occurs. After that, the coal is categorised as bituminous.
Clearly one coal is little heavier like a proper rock, and the other one feels like a booty rock and little lighter. Moments back I’ve told you that as you go more and more inside the Earth, the density of material increases, therefore on my right hand contains a bituminous coal because it’s heavier and it has to settle downwards compared to the lighter one.
- Characteristics of coal mining to coal based all products
Now that we have made a clear distinction as to what is let’s check out its physical characteristics. If you look at the sub bituminous coal, try to see it horizontally, bright and dull bands of coal material are visible, and you see the bright and shiny side. These are well preserved woody material.
Remember that I told you that it is light and feels very; these materials include branches or stents, and the dull side includes minerals that have been washed into the swamp, charcoal that has been formed by fires, or deteriorated plant components. In essence, lignite coal in this form has reached maturity. Geologically speaking, the majority of subbituminous coal comes from the Mesozoic and Cenozoic eras. This call is relatively fragile, which means that it can shatter easily and does so in a concave, cubical shape.
 On the other hand, bituminous coal is a little harder and heavier compared to subbituminous. You will not see any bright band, it’s all dull black. But yet if you remove a little clip of it, you will be able to see the bright band. So, it could be seen this way that with more pressure and heat, the sub bituminous coal becomes a little more intact and goes on to form this piece and this one is going to burn better.
On the other hand, bituminous coal is a little harder and heavier compared to subbituminous. You will not see any bright band, it’s all dull black. But yet if you remove a little clip of it, you will be able to see the bright band. So, it could be seen this way that with more pressure and heat, the sub bituminous coal becomes a little more intact and goes on to form this piece and this one is going to burn better.
Now after this stage comes the anthracite coal, which is very hard, and it has a lot of bright bend all over it. Now I don’t have a piece of anthracite. Overall, what I’m saying is you do understand the consistency of hardness of the coal as it goes from peat that is stage one to anthracite, which is stage five.
 I thought of burning a small chunk of bituminous and subbituminous coal. It is said that both the coal burns easily with a smoky flame of yellow colour. What I’ve noticed is that sub bituminous coal takes a while to heat up whereas bituminous heats up faster due to its high calorific value.
I thought of burning a small chunk of bituminous and subbituminous coal. It is said that both the coal burns easily with a smoky flame of yellow colour. What I’ve noticed is that sub bituminous coal takes a while to heat up whereas bituminous heats up faster due to its high calorific value.
 Coal of different grades and its importance
Coal of different grades and its importance
Coal is a combustible black or dark brown rock that is used as fuel and is primarily composed of carbonised plant materials.
The majority of coals are formed of carbon, but they also contain components like nitrogen, oxygen, sulphur, and hydrogen. The amount of carbon in various forms of coal varies.
Anthracite has more than 92 percent while lignite only includes 60 to 75 percent. A hard glossy material is anthracite. While most types of coal are connected to sedimentary rocks, anthracite undergoes metamorphism and is connected to metamorphic rocks. It is a type of black coal that burns with a blue smokeless flame.
 The world’s largest energy source for generating power is coal, which accounts for almost 70% of China’s electricity. In sum, coal generates around 40% of the electricity used worldwide.
The world’s largest energy source for generating power is coal, which accounts for almost 70% of China’s electricity. In sum, coal generates around 40% of the electricity used worldwide.
Grades of coal
- Coking
- Non coking
- Semi coking
- Easy
- Broken or great coal
sizes of coal
- Normally 2.5 inch minimum to 4-inch maximum mesh
Packaging
- Woven bags weighing 25 kilograms, 50 kilograms, 100 kilograms, 1000 kilograms, bulk containers, or at the buyer’s discretion.
We have excellent relationships with trustworthy and sincere suppliers in the key exporting nations, and we can negotiate deals for new customers wherever they are located.
How the mining industry is responding to tailings dam failures
January 25, 2019, one of the largest and most catastrophic mining related disasters occurred in the Bruma Dino municipality of Brazil. The tailings storage facility fails mine failed, and 11.7 million cubic metres of tailings were released and flooded the local towns and the countryside.
The aftermath was devastating and as of June 2019, 246 People were confirmed dead and 24 missing. So how did this tragedy occur? What can change? Will the mining industry changed to minimise the recurrence of events like this one. First, let’s start with explaining what tailings really are. Tailings are the residual by-product that comes from mineral processing. Mineral processing is the process by which valuable minerals say gold for example, is separated from the waste rock it came from.
Tailings are typically a liquid slurry containing smaller particles of wastewater and are usually stored inside a dam as it is in a liquid form. There are multiple methods of tailings dam construction, and upstream construction was the one used by the mine at Broome. Simply put upstream construction is when the success of dam raises is built inside toward the tailings pond. The benefit of upstream construction is that requires the least amount of film material and therefore is the cheapest and most popular option among mining companies.

The incident investigation is still ongoing to determine the root causes of failure. But five arrests have already been made for those that are responsible Vale is potentially facing a $7 billion fund, which would account for 20% of their 2018 revenues. This incident marked a watershed moment for the industry, leading to the formation of a global safety standard for tailings facilities by the International Council on Mining and Metals (ICMM), the United Nations Environment Programme (UNEP), and the principles for Responsible Investment (PRI).
Alongside the standard of the current practices and suggestions for improvements. The goal is to release the report by the end of 2019 and to take the mining industry one step further in its journey to operate safely and demonstrate transparency.
- Open and close pit mining
Open pit mining is the process of extracting a mineral deposit from the surface, using one or more horizontal benches, and then dumping the waste material, known as overburden and tailings, at a disposal site beyond the final pit’s perimeter. Open pit mining is used to extract both metallic and non-metallic ores. Open pit mining is often differentiated from quarrying on the basis of the material extracted.
 When mining further into the earth, open pit mining is the method of choice for extracting ore from veins and seams that are widely spaced and steeply inclined. The huge expense of filling these pits with all of the waste extracted at the end of the mine’s life would seriously threaten the project’s finances, despite the fact that backfilling normally continues until the pit is complete. Unfortunately, there aren’t many vast, undeveloped areas that could support the construction of such an expensive fence.
When mining further into the earth, open pit mining is the method of choice for extracting ore from veins and seams that are widely spaced and steeply inclined. The huge expense of filling these pits with all of the waste extracted at the end of the mine’s life would seriously threaten the project’s finances, despite the fact that backfilling normally continues until the pit is complete. Unfortunately, there aren’t many vast, undeveloped areas that could support the construction of such an expensive fence.
In spite of its lack of selectivity and mining rate of nearly over 20,000 tonnes per day, open pit mining typically results in high productivity, cheap operating costs, and favourable safety conditions.
 In contrast to underground mining methods, moving a significant amount of overburden outside the mine is necessary during the open pit mining operation. This is due to the fact that ore extraction underneath overburden can only commence after some time has passed since overburden removal began.
In contrast to underground mining methods, moving a significant amount of overburden outside the mine is necessary during the open pit mining operation. This is due to the fact that ore extraction underneath overburden can only commence after some time has passed since overburden removal began.
Open pit mine operations
Open pit mining has as its primary economic objective the recovery of as much marketable mineral concentrate as possible with as little waste as possible. If the mineral is of higher quality, the price will increase. An operation plan outlining the precise steps necessary to extract the ore body from the ground must be drafted in order to keep costs down.
There is a wide range in the size and scale of open pit mines, from tiny, privately held operations that process a few hundred tonnes of ore per day to massive, jointly owned enterprises that remove more than a million tonnes of material each day. The largest mining operations might span dozens of square kilometres. The mining unit operation includes activities such as ripping and dozing, drilling, blasting, loading, and transporting.

- Pollution in Coal mine water
A waterman Australia mine water treatment scheme. This scheme is typical of the mine water treatment schemes that we have across the world. Water is pumped up from a mineshaft of about 120 metres behind us and pumped over to the mine water treatment scheme.
So, the water reaches the top of the scheme. The water in this state is quite high levels of oxidised iron. If we released this water directly into the environment, it could potentially acidify the watercourses because a lot of iron to coat the base of the river. This would make it hard for invertebrates and fish to survive in the river, which is why we treat it.
These are the aeration cascades. This is where we add oxygen in the water to oxidise the ions and encourage it to become a solid. The amount of water we can treat is around 150 litres a second, to put it into some context that means we could treat over five Olympic swimming pools worth of water every day.
The water then flows into the treatment lagoons where the ions finish oxidising, and the solids can settle out. The majority of the ion’s removal happens here. The water then flows into our reedbeds the reedbeds act as a final filter for the removal of the remaining ions solids. They also act as a great habitat for a range of creatures. The treated water then leaves the reed beds in a nice clear state and is ready for discharge. The treated mine water is then discharged into the brook, the treatment ensures that we protected this water course, and the drinking water supplies in the region.
 How do clean mine water
How do clean mine water
I could just explain that there are two shafts, we have a shaft the centre of the site. But just behind it there’s also another main shaft and basically this is an old mine shaft what we use now to collect water just very similar to a well, it’s important that we collect that water, and we pump it out of the mine. Because if that did not happen, that is potential for the museum to flood.
So, every day approximately 10 to 12 million gallons flows into the mine of which 80% of that would naturally flow to wall yard into the old workings. So around two to two and a half million gallons every day, we need to pump out of the mine to ensure that the mine is kept safe, and we keep it clear for public to be able to travel. So, this mineshaft there are two pumps, one pumps 1000 gallons of water every minute, and the other one pumps 600 gallons of water every minute.
So, in a day, we could potentially pump it out 2.4 million gallons, we’re pumping around 2 million gallons a day at the minute because we’re under control with the water and that’s the minimum or the maximum what we need to do so we have got scope to be able to alter the amount what we put.
Now because the water is rainwater or floor water, what runs through the strata, and through all the workings we do get something in the water what we call ochre or your perhaps notice an ochre or commonly known as rust So as the water transfer saw the strata is collected into the water, so as you would see rain fall is clear. By the time it gets into the mine and into the bottom of this shelf, it becomes an orange colour.
 If we put that water out of the mine and just let it flow straight into the river system, we would be breaching the health and safety regulations in terms of what we’re allowed to, to extract and permit into the river system. We talk about the iron or the rust within the water in milligrams per litre of water. So, when we get to our final discharge, the amount what we’re allowed to discharge into the water, and bear in mind, I’m talking now, 2 million gallons of water every day, it cannot exceed three milligrams per litre.
If we put that water out of the mine and just let it flow straight into the river system, we would be breaching the health and safety regulations in terms of what we’re allowed to, to extract and permit into the river system. We talk about the iron or the rust within the water in milligrams per litre of water. So, when we get to our final discharge, the amount what we’re allowed to discharge into the water, and bear in mind, I’m talking now, 2 million gallons of water every day, it cannot exceed three milligrams per litre.
As the water comes up the shaft, it will contain between 20 to 30 milligrams per litre, so, between the raw mine water coming out, and going to discharge, we have got to reduce it by something like 20 to 25 milligrams per litre of water, there is a number of steps that we do to be able to do that, but this is the most important thing at the minute is getting the water out of the mine, getting it into our lagoons, and then we can start the water treatment.
Pours the water comes out of the mine, and we’re talking around 2 million gallons every day comes over the top of this Weir and the reason why it comes over the weir it allows it to go through holes in this way a chamber, it’s oxygenated the water, and then that allows the iron within the water then to separate. The iron dropped into the bottom of this fund is the settling pond and the water what sits on the top then would naturally free flow into some more settling ponds.
The water yolk continues with public concern around 25 milligrams per litre of iron, and on the orange colour, by the time we go through the different elements of the rule, before we get to discharge, then we’ll have it down to a value of three or less.
 So, after the water has left the lagoons, passed through the two lagoons, and then it comes into these concrete settling pads. So, this is just another filtration area where the water comes in. We allow the iron to drop to the floor again, so that that’s sort of where we’re going with the collection point. Then the water was from these four settling ponds into the reinvents
So, after the water has left the lagoons, passed through the two lagoons, and then it comes into these concrete settling pads. So, this is just another filtration area where the water comes in. We allow the iron to drop to the floor again, so that that’s sort of where we’re going with the collection point. Then the water was from these four settling ponds into the reinvents
At this stage, the water comes in between 10 and 15 milligrams per litre of water. After it leaves, we’re probably talking down to eight or 10 milligrams per litre of water. So, the reedbeds then as the effect of reducing it to less than three. So, these are the pour concrete settling ponds, which again take some more rocker out and then goes down to whether
This is our final discharge point. So, the 2 million gallons per day of water pumped out of the mine all finishes up here, we have to clean it to allow it to go into the river system. So, when it first came out to the mine at 20 milligrams per litre, we’re now down to less than till probably two to three milligrams per litre. It looks clean, we took all the nutrients out, we took the iron filings out, we do something different with that now, this is now allowed to go into the norm
Microbes’ clean pollution from abandoned mines
In the massive coal fields. Scars from the mining industry can be seen along its rivers and streams. At its height, coal mining fuelled the industrial revolution, but it also left behind 1000s of mines that continue to pollute the environment with acid mine drainage.

The coal mine drainage is a challenge because so many of the operations that created the problem, have gone bankrupt and aren’t there anymore to deal with and they’ve generated a pollutant source that could last for 1000s of years.
 We have a team of people that includes engineers, hydrologists, microbiologist, to do this important job that they have to clean up acid mines drainage.
We have a team of people that includes engineers, hydrologists, microbiologist, to do this important job that they have to clean up acid mines drainage.
The legacy of the coal mining operations has basically taken rock that was stable under a certain condition and now exposed it to air and water, leading to these reactions that produce this drainage.
This is the airshaft of an abandoned underground coal mine. The orange water coming out of the ground is highly acidic and steeped with toxic heavy metals, principally iron, aluminium, and manganese.
 What happens in coal mines is the pyrite or the iron sulphide minerals that are associated with the coal get oxidised to form sulfuric acid that sulfuric acid dissolves rocks around it and gives you the iron, aluminium, manganese. So, we know some of the metals to look for. The other things that we look for are things like sulphate, we measure the pH, we measure the alkalinity.
What happens in coal mines is the pyrite or the iron sulphide minerals that are associated with the coal get oxidised to form sulfuric acid that sulfuric acid dissolves rocks around it and gives you the iron, aluminium, manganese. So, we know some of the metals to look for. The other things that we look for are things like sulphate, we measure the pH, we measure the alkalinity.
 The challenge is to make acid mine drainage able to support life again and in order for it to support life, we have to raise the pH and we have to get metals to fall out of solution to become precipitates that we can then dispose of safely.
The challenge is to make acid mine drainage able to support life again and in order for it to support life, we have to raise the pH and we have to get metals to fall out of solution to become precipitates that we can then dispose of safely.
In current treatment systems. Acid mine drainage is commonly run through limestone channels to neutralise the acidity and remove heavy metals. The problem is that the limestone becomes coated with iron particles and rendered ineffective needing to be replaced. But the science and engineering team may have found a biological answer near the source of acid mine drainage in a place called the kill zone.
 When you are travelling over landscapes that are affected by acid mine drainage, you often will see pretty large kill zones where there’s no vegetation, there are dead trees, not much life, but the colour is often very red, and that red colour is actually a result of treatment. It’s the result of iron being oxidised and being immobilised in sort of what we’re calling an iron mound, what we find is a whole community of organisms that are together accomplishing this task of removing iron from solution while the pH remains low and that community contains a quite beautiful assemblage of organisms that harvest light, organisms that produce oxygen organisms that cycle iron organisms that recycle organic matter.
When you are travelling over landscapes that are affected by acid mine drainage, you often will see pretty large kill zones where there’s no vegetation, there are dead trees, not much life, but the colour is often very red, and that red colour is actually a result of treatment. It’s the result of iron being oxidised and being immobilised in sort of what we’re calling an iron mound, what we find is a whole community of organisms that are together accomplishing this task of removing iron from solution while the pH remains low and that community contains a quite beautiful assemblage of organisms that harvest light, organisms that produce oxygen organisms that cycle iron organisms that recycle organic matter.
 What we would like to see in the future is a combination of the existing types of passive treatment in which we use natural microbial communities of the sort that are creating iron mounds. Those communities can remove most of the iron and low pH and so that is really a magical ability that we can use as a component of passive treatment to improve their efficiency. But even finding and getting to the many sources of pollution is going to be a challenge.
What we would like to see in the future is a combination of the existing types of passive treatment in which we use natural microbial communities of the sort that are creating iron mounds. Those communities can remove most of the iron and low pH and so that is really a magical ability that we can use as a component of passive treatment to improve their efficiency. But even finding and getting to the many sources of pollution is going to be a challenge.
 What you need to do is break that leaf up into sections, measuring flow and measuring chemistry at different spots across the leaf to figure out and spatially identify where the worst pollutant sources might be coming in. Then ideally, if there is assistance that we can provide with helping in a growing greener grant for remediating some of these sources, that’s what we want to do in the future.
What you need to do is break that leaf up into sections, measuring flow and measuring chemistry at different spots across the leaf to figure out and spatially identify where the worst pollutant sources might be coming in. Then ideally, if there is assistance that we can provide with helping in a growing greener grant for remediating some of these sources, that’s what we want to do in the future.
Clean Coal Technology
We just heard coal is dirty. But you might have heard politicians or television commercials talking about clean coal technology, what’s going on? Those guys probably want you to think about coal without CO2 emissions. But the fact is, there are no coal power plants without CO2 emissions in the US or anywhere else.
There are a few pilot projects to add units onto existing coal plants to capture carbon. There are technologies proposed to turn coal into gas, separating the CO2 before it is burned. But both of these are too experimental and expensive to roll out at the huge commercial scales necessary to make a difference today.
There are however technologies to reduce the other pollutants, what’s called the bag house, like rows of giant vacuum cleaner bags, remove all the ash and some of the heavy metals. Scrubbers and catalysts capture sulphur oxides, nitrogen oxides, and some of the mercury. The best coal plants capture these emissions, but worldwide, more of them don’t. Why?
Because adding these processes is complicated and expensive that takes away some of the biggest benefits of coal, simplicity, and affordability. It takes energy to run all these processes and that comes from the coal plant itself, which means to produce the same amount of electricity, the plant has to burn more coal, and that produces more pollutants and more CO2. So, we can clean up particulates and other pollutants from coal plants but with greater cost, complexity and carbon emissions.
- Coal to liquids chemical Process
In this portion we want to illustrate the common operations involved using a coal to liquid process.
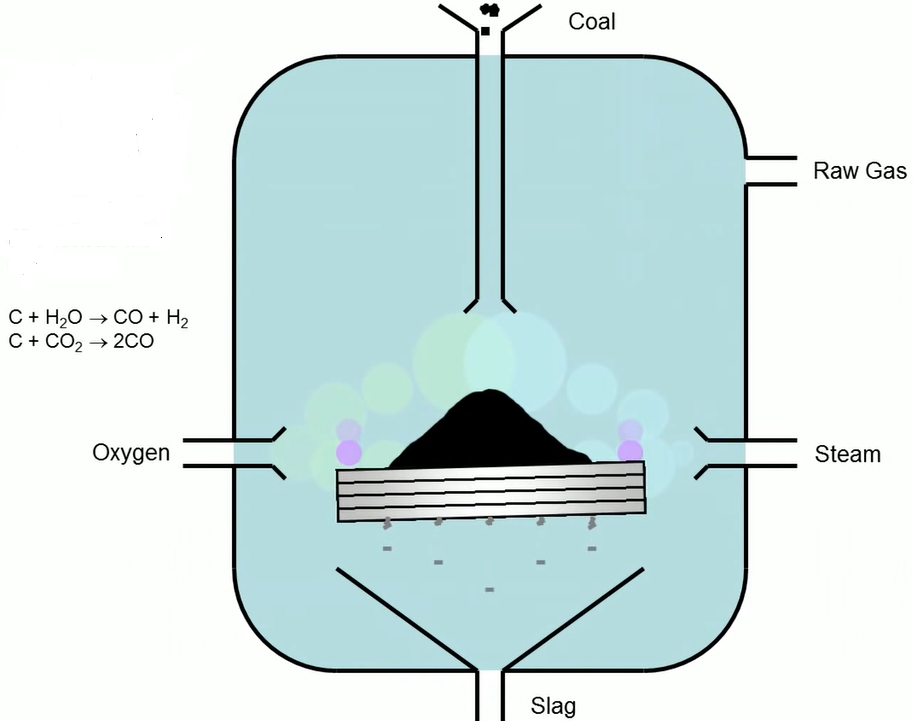
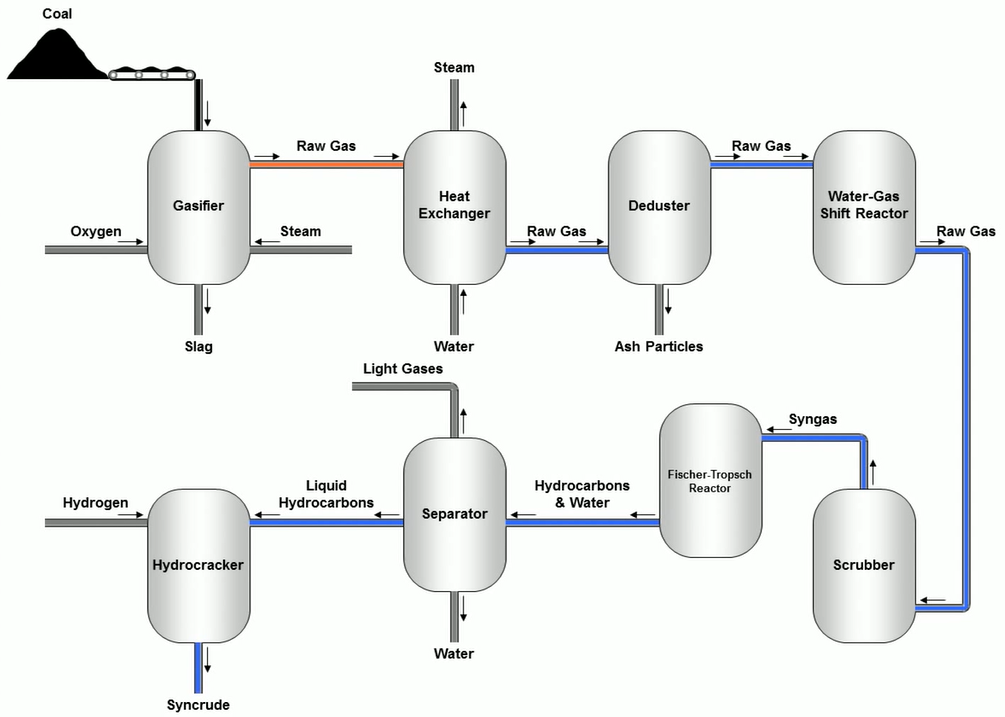 In the first steps, the oxygen, steam and coal are sent into a gasifier for gasification. In a gasifier, the major component of coal, carbon, combines with steam and oxygen to produce primarily carbon monoxide and hydrogen, which then creates the raw gas that comes out the top.
In the first steps, the oxygen, steam and coal are sent into a gasifier for gasification. In a gasifier, the major component of coal, carbon, combines with steam and oxygen to produce primarily carbon monoxide and hydrogen, which then creates the raw gas that comes out the top.
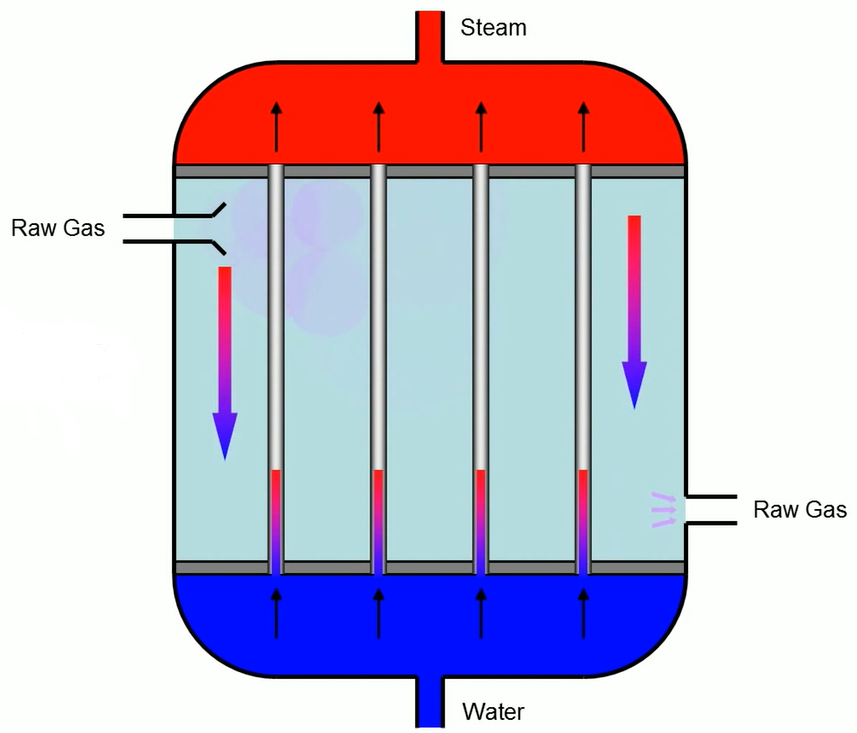
 The heat exchanger receives the raw gas. Water that has been preloaded on a counter in the exchanger row is used to cool down the gas while also producing steam from the water.
The heat exchanger receives the raw gas. Water that has been preloaded on a counter in the exchanger row is used to cool down the gas while also producing steam from the water.
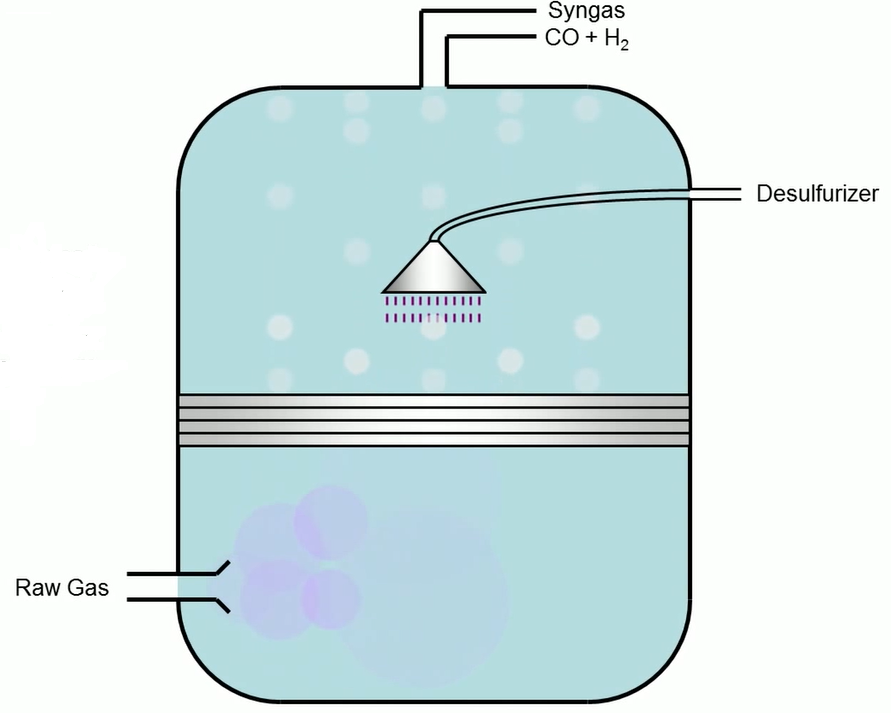
 In the third step, raw gas is turned into a de-duster. Ash particles suspended in raw gas removed through the de-duster. After deducting the road gas essential water gets shipped the reactor. The water is transported to the reactor, which adjusts the hydrogen to carbon monoxide ratio. Carbon monoxide or water vapour react in a reactor to produce carbon dioxide and hydrogen.
In the third step, raw gas is turned into a de-duster. Ash particles suspended in raw gas removed through the de-duster. After deducting the road gas essential water gets shipped the reactor. The water is transported to the reactor, which adjusts the hydrogen to carbon monoxide ratio. Carbon monoxide or water vapour react in a reactor to produce carbon dioxide and hydrogen.
 The scrubber receives the raw gas after that. Syn gas is the name given to a mixture of hydrogen and carbon monoxide that has been elevated and cleaned in a scrubber in the south. Fischer Tropsch reactor is filled with syngas. Carbon monoxide and hydrogen are catalytically transformed into long chain paraffins in the Fischer Tropsch reactor using syn gas. This is how people respond when coal is turned into liquids.
The scrubber receives the raw gas after that. Syn gas is the name given to a mixture of hydrogen and carbon monoxide that has been elevated and cleaned in a scrubber in the south. Fischer Tropsch reactor is filled with syngas. Carbon monoxide and hydrogen are catalytically transformed into long chain paraffins in the Fischer Tropsch reactor using syn gas. This is how people respond when coal is turned into liquids.
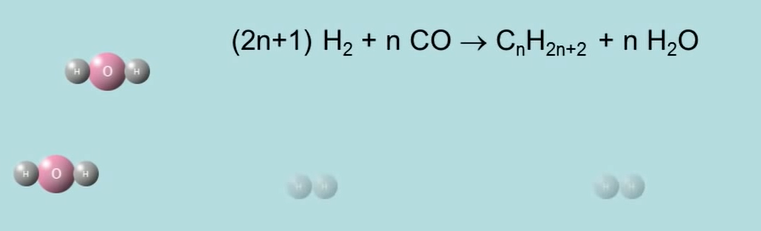
 Water and hydrocarbons from production are transferred to a separator. Light gases, liquid hydrocarbons, and waters are separated from the mixture when it is refined using a separator.
Water and hydrocarbons from production are transferred to a separator. Light gases, liquid hydrocarbons, and waters are separated from the mixture when it is refined using a separator.
The hydrocracked is the final phase. Long chain earrings are heated in the hydrocracker and react with hydrogen to produce short chain materials like jet fuel, diesel, and gasoline. The crudest thing is the store, to finish.
- Coal gasification
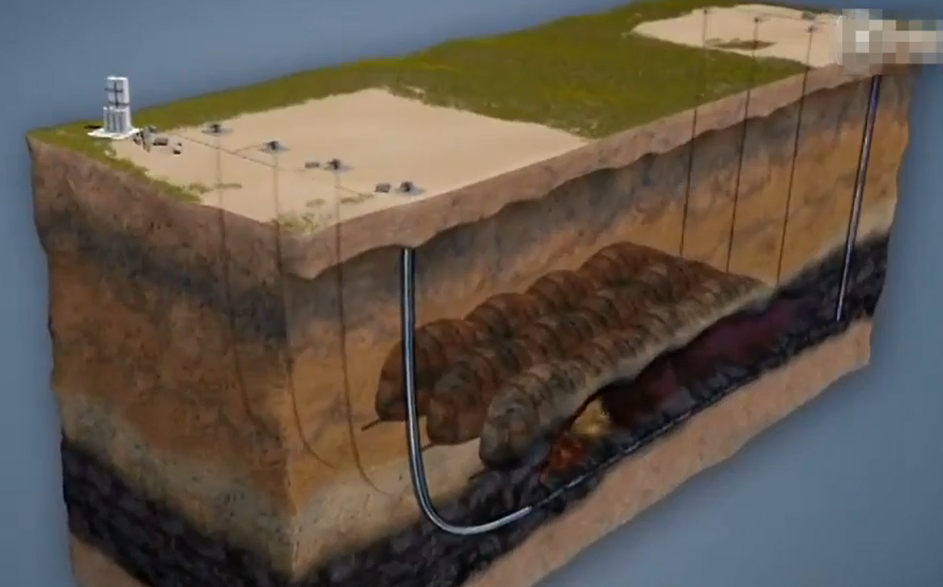
Synthetic natural gas, synthetic gas, and power generation may all be made easily from synthetic gas. Deep holes that are unsuitable for conventional mining can now be turned into lucrative goods thanks to UCG. UCG simply works. More than 300 meters and up to two kilometres below the surface, a vertical production well is dug into the coal seam.
Steel casing and specialized high-temperature cement are erected from the surface to the coal seam once the well has been drilled to assure isolation from any groundwater aquifers.
The production well is subsequently intersected by the injection well, which is then dug horizontally in the coal seam. In order to maximize the amount of coal converted into gas, the well is drilled near the base of the coal seam.
Before the gasifier is turned on, a coil tubing device is set up over the injection well. The oxygen supply system and surface infrastructure are both connected to the injection well. The surface facilities at UCG are built in a modular manner.
An ignition tool is attached to the coil tubing and put into the injection well to initiate the UCG process. After passing through the injection well, the ignition tool is placed in a ready-to-ignite position. The coal gasification process is initiated within a few hours by the heat produced by the igniting equipment and the oxygen injected from the surface. Oxygen is pumped into the well in high volumes to maintain gas production.
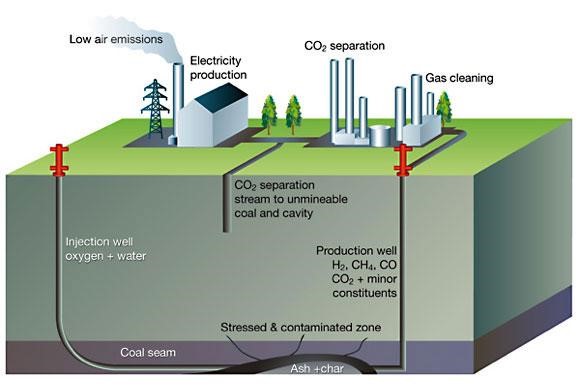 During the gasification process, steam and coal react to produce carbon monoxide and hydrogen. In order to guarantee that the high temperature zone is contained within the coal seam, the UCG side made very careful decisions. The ash-like by-products of the UCG process are left in the coal seam. The Syn gas, which has a high concentration of carbon monoxide, hydrogen, and methane, travels from the gasification chamber up the production well and through the horizontal connection in the coal seam.
During the gasification process, steam and coal react to produce carbon monoxide and hydrogen. In order to guarantee that the high temperature zone is contained within the coal seam, the UCG side made very careful decisions. The ash-like by-products of the UCG process are left in the coal seam. The Syn gas, which has a high concentration of carbon monoxide, hydrogen, and methane, travels from the gasification chamber up the production well and through the horizontal connection in the coal seam.
Synthetic gas is sent to a neighbouring plant where it is transformed into chemicals, liquid fuels like diesel and jet fuel, or electricity. All of the coal in the area of the well is converted into syn gas by repeating the procedure throughout the length of the injection well.
In order to generate the necessary sin gas and generate extremely large amounts of energy from a small footprint, numerous gasifiers are run concurrently in commercial operations. UCG has various advantages, such as access to deep stranded coal without the requirement for mining, which uses more energy than other processes and causes less surface disturbance and no underground labour. as soon as UCG activities are over. After then, the site is carefully decommissioned.
- Wastewater Treatment Solutions for the Mining Industry
Water is basic, natural, pure a symbol of life and wellbeing. We must keep it that way. Water plays an essential role in the mining industry. Take the gold mining industry. The industry’s biggest difficulty is finding a long-term, affordable solution for the safe disposal of wastewater.
To avoid contaminating nearby groundwater, the extraction process’ hazardous by-products and volatile compounds must be neutralised. Acid mine drainage poses a serious danger to the quality of the water, and conventional remedies such Brian streams and tailing dams are prone to leakage.
Through the provision of an ongoing, fully integrated water treatment system for the mining industry, Waterman Australia has made a significant advance. Waterman Australia currently has five active mining operations that treat every conceivable contaminant in an online system while running continuously at 10 million litres per day per site.
 The acid mine drainage (AMD) issue has been completely solved using Waterman Australia’s cutting-edge technology of mining effluent treatment plants. Without the use of chemicals or membranes, the company’s ground-breaking proprietary technique enables the removal and annihilation of all amounts of dangerous heavy metals, arsenic sulphates, and cyanide. Some of the major firms in the mining industry proudly use the services of Waterman Australia.
The acid mine drainage (AMD) issue has been completely solved using Waterman Australia’s cutting-edge technology of mining effluent treatment plants. Without the use of chemicals or membranes, the company’s ground-breaking proprietary technique enables the removal and annihilation of all amounts of dangerous heavy metals, arsenic sulphates, and cyanide. Some of the major firms in the mining industry proudly use the services of Waterman Australia.
We discover a lot of in fact heavy metals in our water bodies. Most recently my concern was with mercury but a lot of arsenic and other heavy metals. Waterman Australia doing the technology they are using. The most advanced technologies so far. They are up to the task they have the capability of cleaning wastewater to the level that if this will stuff into the environment is safe you know for our people, and they have our people.
 Traditional solutions emphasise containment while our solutions offer the mining industry two options, the safe discharge of the water back to the environment or the return of the treated water for reuse. Chemicals and expensive reverse osmosis membranes are not required thanks to Waterman Australia technology. This makes the removal of all cyanide, heavy metals, arsenic, and sulphate forms more sustainable and, most importantly, more affordable.
Traditional solutions emphasise containment while our solutions offer the mining industry two options, the safe discharge of the water back to the environment or the return of the treated water for reuse. Chemicals and expensive reverse osmosis membranes are not required thanks to Waterman Australia technology. This makes the removal of all cyanide, heavy metals, arsenic, and sulphate forms more sustainable and, most importantly, more affordable.
 Waterman Australia localised support teams provide an immediate response to your needs. within 24 hours we can provide you with a comprehensive tailor-made plan to meet your requirements. Within seven months your customised system is up and running 24/7. There is no limitation to the size of treatment volumes.
Waterman Australia localised support teams provide an immediate response to your needs. within 24 hours we can provide you with a comprehensive tailor-made plan to meet your requirements. Within seven months your customised system is up and running 24/7. There is no limitation to the size of treatment volumes.
Water and Abandoned Metal Mines projects
The project is aiming to identify the sources of pollution and find solutions to reduce the impact of metal mines to benefit the environments such as your river ecology, and also the economy by removing a lot of the metal rich sediment which is travelling down.
The debris from the dressing floor of the mines was thrown next to the river, forming a sizable spoil heap with extremely steep slopes. Over time, this has recovered and sort of degraded, and more and more materials are slipping into the stream evidently causing contamination.
 In order to solve this problem, we connected the two culvert pieces and kept them apart from the stream. With the slopes now at a safe angle, they can sort of maintain them going forward and stop more diffuse pollutants from entering the watercourse.
In order to solve this problem, we connected the two culvert pieces and kept them apart from the stream. With the slopes now at a safe angle, they can sort of maintain them going forward and stop more diffuse pollutants from entering the watercourse.
With copies frequently tending to fall when the river cleaned up as a result of the activities, it would assist to increase the types of quantities of fish and aquatic life in the ecosystem to vote making many modest improvements that will add up to a significant impact.
Sometimes we forget the sort of legacy sort of mine energy when the mine stops the problems don’t stop you know, the sort of go on to the future, obviously, is quite a large challenge of addressing all of these.
 What we’re looking to do is to reduce that pollution for the benefit for the environment and it reduces the amount of sediment the tiny estuary which can build up in the ship and birth so it can help with that respect as well.
What we’re looking to do is to reduce that pollution for the benefit for the environment and it reduces the amount of sediment the tiny estuary which can build up in the ship and birth so it can help with that respect as well.
 I think for quite many years it’s been fine nothing some large storm started to erode the bank so bringing the water in contact with the mine waist and those two then that releases the diffuse metal pollution into the watercourse.
I think for quite many years it’s been fine nothing some large storm started to erode the bank so bringing the water in contact with the mine waist and those two then that releases the diffuse metal pollution into the watercourse.
The metals have a negative impact on the river ecology, so your river flies on fish populations, it’s also transporting a lot of metal rich sediments down through to the south time. The main sources of metals in the watercourse are cadmium, lead, and zinc.
 So, we need to put works in place to separate those flows. So, we can then monitor the individual flows properly and find out where the worst contamination is coming from and then the water then runs straight across the existing access track. So, we need to make that physically stable, so the water goes back underneath the track again in a proper culver and then we’re putting in place a concrete Canvas line channel to hold the water in its correct alignment and transport itself straight down to the bottom of the valley without coming into contact with contaminated materials and without causing erosion.
So, we need to put works in place to separate those flows. So, we can then monitor the individual flows properly and find out where the worst contamination is coming from and then the water then runs straight across the existing access track. So, we need to make that physically stable, so the water goes back underneath the track again in a proper culver and then we’re putting in place a concrete Canvas line channel to hold the water in its correct alignment and transport itself straight down to the bottom of the valley without coming into contact with contaminated materials and without causing erosion.
There are a number of constraints to the whole works around environmental issues, including the rare plant species and the lichens. The boulders that were found near the river had rare lichens identified on them and they’ve been placed away from the river so they can continue to be undisturbed.
 Cleaning up rivers polluted by historical metal mining
Cleaning up rivers polluted by historical metal mining
We got a long industrial history of mining for minerals. But unfortunately, although most of the mines closed at the end of the 19th century, we still have over one and a half 1000 kilometres of river that are heavily polluted by metals such as cadmium, lead and zinc and these cause harm to fish and other wildlife in rivers.
What we’re trying to do through the water and abandoned metal mines programme, which was set up in 2021, is to clean up this industrial legacy. If we’re able to clean up all these rivers, we will deliver hundreds of millions of pounds in benefit for people and the economy and helps us to deliver the government’s 25-year plan to improve the environment.
 The first track mine was a leaden zinc and varieties mine that operated from 1835 until 1991. But unfortunately, the metals that were coming out of the mine in the mine water, we’re polluting about 10 kilometres of the river, which is a special area of conservation. It’s very, very important part of the Lake District.
The first track mine was a leaden zinc and varieties mine that operated from 1835 until 1991. But unfortunately, the metals that were coming out of the mine in the mine water, we’re polluting about 10 kilometres of the river, which is a special area of conservation. It’s very, very important part of the Lake District.
The treatment system that we had designed for us is the first time this technology has been used, it’s really exciting because it uses natural processes to move more than 90% of the metals from the mine water simply by passing the mine water down through a compost layer, which strips out the metals and then you end up with a clean water that comes out of the bottom.
 We don’t need to add any chemicals that’s it’s much cheaper to operate than other types of schemes and it’s been really, really effective. It’s a very special site in the Lake District National Park and a scheduled ancient monument because of the long history of mining there. We were able to use some of the old waste ponds that the miners had operated to hold the treatment ponds now, which meant that we’re now able to help to write the next chapter in the long industrial history, as well as cleaning up the river for wildlife people.
We don’t need to add any chemicals that’s it’s much cheaper to operate than other types of schemes and it’s been really, really effective. It’s a very special site in the Lake District National Park and a scheduled ancient monument because of the long history of mining there. We were able to use some of the old waste ponds that the miners had operated to hold the treatment ponds now, which meant that we’re now able to help to write the next chapter in the long industrial history, as well as cleaning up the river for wildlife people.
In 1999, the Salton burn stream turned bright orange overnight, and the orange colour carried on into the North Sea over a very popular surfing and bathing water beach and this is because the mines gradually filled up with rainwater, and that started washing out really, really iron rich water out of the ground. So, after several years of developments in 2015, we built the salt burn mine water treatment scheme.
 The water treatment system works by pumping the mine water out of the ground, and that water is then passed over a series of cascades into a settlement pond and that puts oxygen into the mine water that takes out most of the iron and then we take the fairly clean water through a large wetland that we had built local reads of flag iris and other water flowers and that takes out the last bits of iron from the mine water.
The water treatment system works by pumping the mine water out of the ground, and that water is then passed over a series of cascades into a settlement pond and that puts oxygen into the mine water that takes out most of the iron and then we take the fairly clean water through a large wetland that we had built local reads of flag iris and other water flowers and that takes out the last bits of iron from the mine water.
 It’s great for bird life and wild fowl. And we have also put in some solar panels as a means of lowering the carbon footprints of this treatment scheme. Using the solar panels is a cleaner way of driving those pumps. Within a year of the treatment scheme starting operating had fish returning to the river and the river is now clean, whereas before it looked very much like tomato soup, and we estimate this will deliver about 10 million pounds of economic benefit to the community over the next 25 years.
It’s great for bird life and wild fowl. And we have also put in some solar panels as a means of lowering the carbon footprints of this treatment scheme. Using the solar panels is a cleaner way of driving those pumps. Within a year of the treatment scheme starting operating had fish returning to the river and the river is now clean, whereas before it looked very much like tomato soup, and we estimate this will deliver about 10 million pounds of economic benefit to the community over the next 25 years.
In January 1992 There was a massive outbreak of 50 million litres of highly acidic water containing enormous amounts of cadmium, zinc, arsenic and copper, which polluted several kilometres of the river and turn the FAL estuary bright orange with the iron that was in that mine.
The current treatment system was built in 2000s and it works by having 10 large pumps which keep the water level in the mine below the river level, that means that there’s no water getting into the river from the mine workings. The treatment system works by taking that water and mixing it with line and other chemicals.
 The waste sludge is put into a tailings pond that was originally built in the 1970s when the mine was operating, and the Clean Water goes back into the Cannon River. Unfortunately, although the wheel drain treatment plant is incredibly effective, that river cannon is still the most metal polluted river in England and that’s because of lots of other sources of metals in that catchment. And one of the key sources there is the 65 kilometre long, great county addicts that drains most western Cornwall, and we’re looking at ways that we can begin to clean that up, as well as all the other sources.
The waste sludge is put into a tailings pond that was originally built in the 1970s when the mine was operating, and the Clean Water goes back into the Cannon River. Unfortunately, although the wheel drain treatment plant is incredibly effective, that river cannon is still the most metal polluted river in England and that’s because of lots of other sources of metals in that catchment. And one of the key sources there is the 65 kilometre long, great county addicts that drains most western Cornwall, and we’re looking at ways that we can begin to clean that up, as well as all the other sources.
 We think we have about 100 schemes we need to build over the next few years and we are developing 20 or 30 schemes at the moment which we ready to build over the next sort of five to 10 years. If we don’t take action to clean up this type of pollution. It’ll continue to cause damage to the environment for hundreds more years.
We think we have about 100 schemes we need to build over the next few years and we are developing 20 or 30 schemes at the moment which we ready to build over the next sort of five to 10 years. If we don’t take action to clean up this type of pollution. It’ll continue to cause damage to the environment for hundreds more years.

- Coal chemicals wastewater and other products
Making Paint from Coal Mine Waste Could Clean Up Streams
After a coal mine shuts down, the pollution doesn’t stop. Toxic waste flows for decades, contaminating rivers and killing aquatic life. And it’s nearly impossible to contain. For a long time, most of these streams were just written off.
 Now a team has developed a method to harvest the muck and turn it into pain, but can it really clean up a century of worldwide waste? our team built a system of pipes that collect smelly goo called acid mine drainage or AMD for short. It’s highly acidic water leaking from an old coal mine that closed over 100 years ago, when you leave a mine, and you just walk away from it. What happens is it fills up instantly with water, there really is no way to seal the mines completely. The water that still leaks from mines today creates iron oxide, which can be lethal to aquatic life.
Now a team has developed a method to harvest the muck and turn it into pain, but can it really clean up a century of worldwide waste? our team built a system of pipes that collect smelly goo called acid mine drainage or AMD for short. It’s highly acidic water leaking from an old coal mine that closed over 100 years ago, when you leave a mine, and you just walk away from it. What happens is it fills up instantly with water, there really is no way to seal the mines completely. The water that still leaks from mines today creates iron oxide, which can be lethal to aquatic life.

That pipe is full of iron sludge and it’s going to come out of there really fast. But it also happens to be an essential ingredient to make paint.

The team filters buckets of iron oxide using these troughs. Smooth it out and put some cool whip on there and it’s like pumpkin pie. It’s a bit more than this Mindsight pumps out in a single day. Then they move it to their research facility where engineers wash the pigment to remove impurities that affect the final colour. Basically, we’re just diluting out all the dissolved solids.
 The team dries the pigment before shipping it off to a giant kill. They change the colour by controlling the temperature. Then they send the pigment to Portland, Oregon, where Gamblin artists colours uses it to make paint.
The team dries the pigment before shipping it off to a giant kill. They change the colour by controlling the temperature. Then they send the pigment to Portland, Oregon, where Gamblin artists colours uses it to make paint.
We wanted to be the first to make colour with it. We were just kind of all in. There are three colours that are all made from pigment that has been painstakingly reclaimed by team. This one is called Iron violet. This worker mixes the pigment, zinc and flax oil and the mill uses heat and pressure to combine the pigment in oil So with a little bit of pressure, we draw it down.
Then workers test for thickness, texture, and colour. Finally, they bottle it up Gamblin markets to paint as reclaimed Earth colours.
The circle gives them permission to decide for themselves instantly. Whether that is a universe a planet, a stream or microbes and the new colours are taking off. Painters across the country have shared what they’ve created using the hashtag reclaimed colour. For now, true pigments can’t harvest enough iron oxide to clean up an entire stream.
 But the company plans to scale up. By 2024 True pigments plans to open a larger facility. It will harvest iron oxide and clean the water at one of Ohio’s most polluted acid mine sites. That would mean the company could harvest raw material that could be used in all kinds of products, not just paints,
But the company plans to scale up. By 2024 True pigments plans to open a larger facility. It will harvest iron oxide and clean the water at one of Ohio’s most polluted acid mine sites. That would mean the company could harvest raw material that could be used in all kinds of products, not just paints,
Construction materials, concrete bricks. It’s used for a lot of industrial coatings, agricultural fertiliser cosmetics, we really had to find something useful to make out of this really, you know detrimental pollution.
our team’s method of retrieving iron oxide is more sustainable than mining it. Unfortunately, they’re dealing with a nearly unlimited resource. There are nearly 10,000 square miles of abandoned coal mines across the United States.
Iron oxide didn’t do anything wrong. Iron oxide is not the blame here. If you treat it right like we’re doing it is an asset. It is beautiful. These mines will continue producing AMD for hundreds of years.
Rescuing rare earths from coal mine waste
This is West run a slow-moving Creek lined with honeysuckle and wild rose that meanders through the mountains of West Virginia. The tranquil scene near Morgantown is almost picture perfect but look carefully at the colour of the sediment that lies beneath the water.
Fire orange on one side, milky white on the other. This is not a healthy Creek. In fact, water quality experts say this creek is dead. What killed West run is acid mine drainage from coal mines. Acidic mine water is tainting many streams and the heart of West Virginia’s coal country. But this isn’t just a story about drainage and pollution. It’s also about recovery in more ways than one.
 And acid mine drainage is something we’ve been trying to make go away for a very long time here. And we’ve actually been on your halo river that you crossed on the way into town here. It used to be orange only in the early 1970s. It’s now a fishery and the streams that feed into it which were also orange to a large extent where we’re sort of moving the Clean Water zone and all these streams way up toward the headwaters or just where the mines actually are.
And acid mine drainage is something we’ve been trying to make go away for a very long time here. And we’ve actually been on your halo river that you crossed on the way into town here. It used to be orange only in the early 1970s. It’s now a fishery and the streams that feed into it which were also orange to a large extent where we’re sort of moving the Clean Water zone and all these streams way up toward the headwaters or just where the mines actually are.
Over the past several decades, the state of West Virginia has started treating the discharge from many shuttered coal mines, such as the Omega mine shown here, and active mines are required to treat acid mine drainage with lime or some other neutralising agent to prevent streams and rivers from ending up like West run.
 If acid mine drainage is not treated, it corrodes bridges, harms aquatic life, and contaminates water, giving it a rusty flavour. The stream’s earlier discolouration is likewise a result of it. From mines, acid mine drainage results. when the coal is highly pyrite or iron sulphide enriched.
If acid mine drainage is not treated, it corrodes bridges, harms aquatic life, and contaminates water, giving it a rusty flavour. The stream’s earlier discolouration is likewise a result of it. From mines, acid mine drainage results. when the coal is highly pyrite or iron sulphide enriched.
Iron to combines with oxygen and hydroxides to make iron three ferric hydroxides, which precipitate as orange solids. The pyrite reacts with oxygen and water to produce sulfuric acid and iron to or ferrous iron between pH three and 3.5. High concentrations of aluminium and manganese, which precipitate as hydroxides, are also frequently seen in acid mine drainage. The milky white sediment in West Ron is caused by aluminium hydroxide. Acid mine drainage must be treated at the source in active mines according to regulations.
Tonnes of sludge with a predominance of iron, aluminium, and manganese hydroxides are left over after treating acid mine drainage. Usually, big ponds or dewatering containers like the TENCATE Gu tubes in this picture are used to sun-dry the sludge. The sludge is frequently buried after it has dried. A significant portion—roughly half—of the expenses related to treating acid mine drainage are related to disposing of the sludge.
 Although it’s too early to tell whether web use extraction process will be economical. The technical performance looks promising is in cabbage new from data collected in the 1990s by the US Geological Survey. That acid mine drainage entering treatment plants contains rare earth elements, but the water leaving the plants does not.
Although it’s too early to tell whether web use extraction process will be economical. The technical performance looks promising is in cabbage new from data collected in the 1990s by the US Geological Survey. That acid mine drainage entering treatment plants contains rare earth elements, but the water leaving the plants does not.
The question then became how to recover the valuable rare earths from the sludge material. The WVU team is still optimising that process, but here’s the gist of it.
The first step is to digest the sludge in a tank of acids such as sulfuric acid. Based on what we’ve seen in the laboratory, we can get somebody 90 95% of all of the rare earth out of sludge by adjusting the pH down to about one.
The pregnant leach solution, an acidic solution, then enters a set of 100 columns known as mixer settlers. An organic phase, such as kerosene, and an extract that binds to rare earth elements are both present in the first set of containers. This results in the formation of an emulsion with rare earths in the organic phase. The researchers utilise a powerful acid to return the rare metals to the aqueous phase at a higher concentration than when they first started, after eliminating undesirable elements from it. After raising the pH, the team observes the rare earths precipitate as a clean sludge.
 Not all rare earth elements are created equal and some of them are very valuable and some of them are not. So, one of the things we’ll be doing in this process is optimising recovery of the valuable rare earth elements and not waste time and money. Find extracting things that aren’t very useful.
Not all rare earth elements are created equal and some of them are very valuable and some of them are not. So, one of the things we’ll be doing in this process is optimising recovery of the valuable rare earth elements and not waste time and money. Find extracting things that aren’t very useful.
Rare earths are needed for many modern-day commodities, and the US is keen to have a ready source of them at hand for national security reasons.
as the high-tech sectors expanded. Demand for rare earth elements has increased along with it. Unfortunately, we cannot obtain rare earth elements domestically. As a result, we mostly import rare earth materials from the People’s Republic of China. And at that point, it turns becomes a matter of household significance.
The US Department of Energy concurs that having a local source of rare earth elements is crucial for national security, especially in light of the ongoing trade spat between the US and China.
 Decarbonization and the Mining Industry
Decarbonization and the Mining Industry
It is Surprise that global carbon dioxide emissions are on the rise. It is estimated that since 1850, emissions have risen more than 30%. To limit global temperature increases, mining companies are working hard toward decarbonisation. Contrary to popular belief. Many mining comes and he’s cared for and invest in protecting the environment. To support decarbonisation. Some companies are adding renewables to the energy supply for developing new equipment technologies that releases the less greenhouse gases.
 Let’s look at a few examples of these carbon reduction initiatives.
Let’s look at a few examples of these carbon reduction initiatives.
Energy supply
First up is energy supply. Since many mining operations are dependent on diesel generators for energy, those in the right climate conditions are turning to solar energy to help power their operations.
 Many operations in South Africa and Australia are already installing solar panels along with the energy storage equipment. For example, one mining company installed a one-megawatt solar array in 2012 that replaced the need for 450,000 litres 120,000 gallons of diesel and reduced CO2 emissions by 2000 tonnes every year. Another decarbonisation pathway is the electrification of equipment, which aims to replace the diesel equipment that most mining companies are currently using. In underground mines, ventilation is needed to keep fresh air in the working areas. Besides the direct benefit of eliminating diesel electrification can reduce noxious fumes and underground mines, which will reduce the ventilation power demands.
Many operations in South Africa and Australia are already installing solar panels along with the energy storage equipment. For example, one mining company installed a one-megawatt solar array in 2012 that replaced the need for 450,000 litres 120,000 gallons of diesel and reduced CO2 emissions by 2000 tonnes every year. Another decarbonisation pathway is the electrification of equipment, which aims to replace the diesel equipment that most mining companies are currently using. In underground mines, ventilation is needed to keep fresh air in the working areas. Besides the direct benefit of eliminating diesel electrification can reduce noxious fumes and underground mines, which will reduce the ventilation power demands.
 If this is achieved, the company expects to reduce 7000 tonnes of CO2, 2 million litres of diesel and 1 million litres of propane consumption altogether. The pathway to decarbonisation is a difficult one, as they face technical, logical, and financial challenges to implement their innovation.
If this is achieved, the company expects to reduce 7000 tonnes of CO2, 2 million litres of diesel and 1 million litres of propane consumption altogether. The pathway to decarbonisation is a difficult one, as they face technical, logical, and financial challenges to implement their innovation.
- Coal based power generating energy
Coal is used to produce electricity.
Coal is used in a boiler at a thermal power plant, such as the border dam power plant, to turn water into steam. A shaft is turned by a turbine that receives steam. A generator that generates power when the shaft revolves is attached to it.
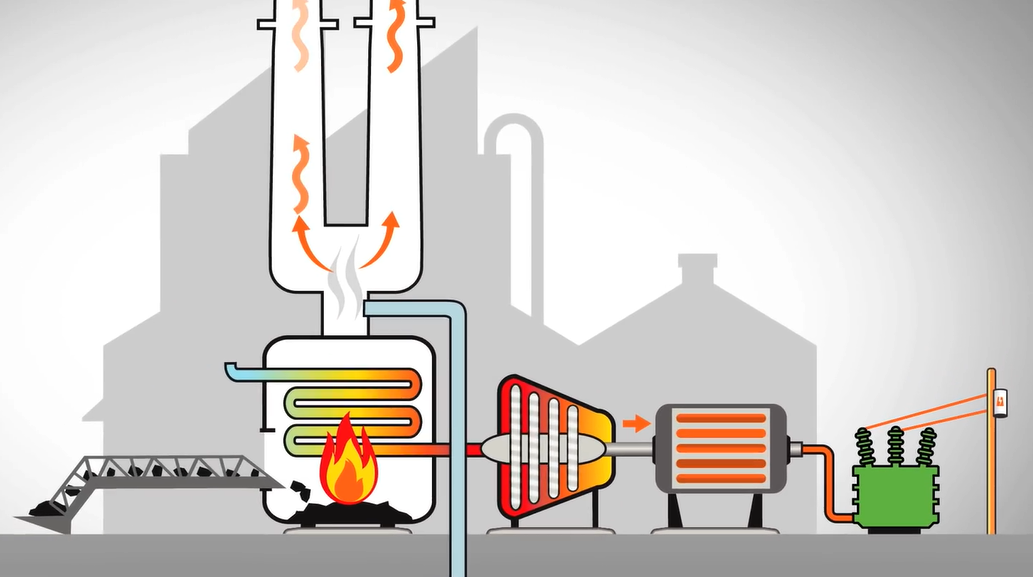 The used steam from the turbine is transformed back into water by a condenser and then utilised in the boiler. One of the first power plants in the world to incorporate carbon capture and storage is this one. To lessen our contribution to climate change, we permanently bury the compressed carbon dioxide that is released by one of our coal-burning units.
The used steam from the turbine is transformed back into water by a condenser and then utilised in the boiler. One of the first power plants in the world to incorporate carbon capture and storage is this one. To lessen our contribution to climate change, we permanently bury the compressed carbon dioxide that is released by one of our coal-burning units.
How do you generate electricity using coal
Over 4 million residential, commercial, and industrial clients receive nearly 70 million megawatt hours of electricity annually from the 20 power facilities that First Energy operates across the globe. Coal is used to produce more than half of first energy’s electricity, compared to around 60% nationwide.
Let’s examine the process used to produce electricity in coal-fired power plants similar to those run by First Energy. Three producing units at this plant can generate more than 2000 megawatts of power. When operating at maximum efficiency, one single power plant can generate enough electricity to meet the demands of 1.5 million households and businesses.
Coal Arrival or Coal yard
A fuel source is necessary for the production of electricity. Coal is used as fuel for this facility; it is delivered primarily by barge, but also by rail and truck. 1500 tonnes of coal, or enough to run the plant for a few hours, are delivered by each barge. Over a million tonnes of coal are stacked adjacent to the facility in a structure known as a stacker since the plant needs about 21,000 tonnes of coal per day. A conveyor that is a quarter of a mile long and can carry up to 900 tonnes of coal into the plant every hour uses a reclaimer to scoop coal onto the conveyor.
Storing and Pulverizing
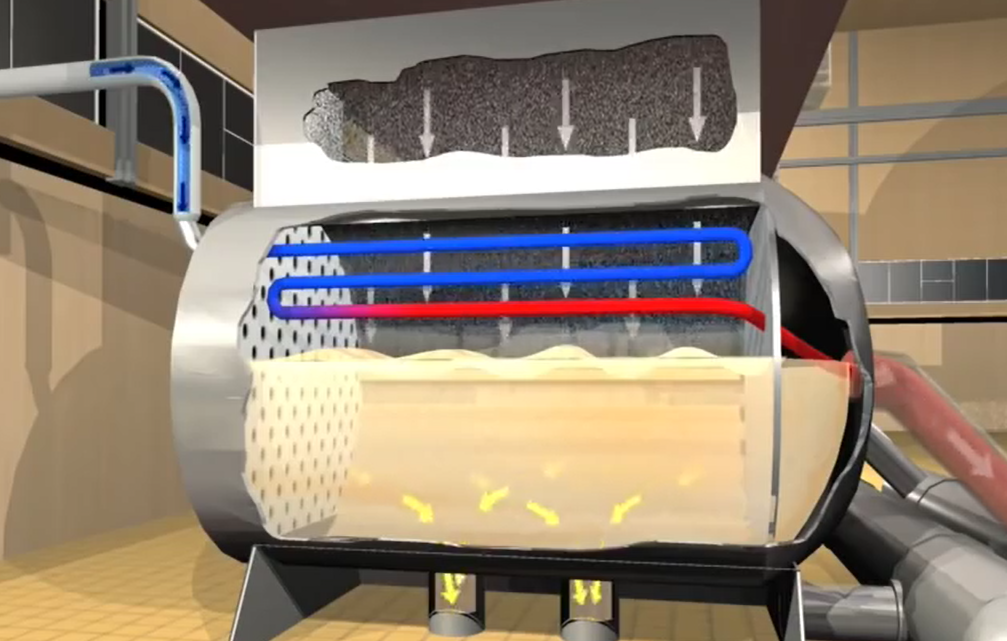
Coal can be kept in bunkers inside the facility for up to 30 hours. Bunkers deliver coal to feeders, where it is weighed and transported to pulverisers. Eight pulverisers are supplied by 16 coal feeders in each generating unit.
A massive drum that contains hundreds of steel balls spins as the coal enters the pulveriser, where it is ground into a fine powder. The coal exits the pulverisers and moves to the boiler, where we will find powder. Coal may be pulverised at a rate of about 335 tonnes per hour.
 Combustion and Steam
Combustion and Steam
The powdery coal is heated by large fans before being blown into the boiler. Miles of tubes loaded with high-quality water run throughout the boiler. When the coal burns within the boiler, it releases energy and creates extreme heat, which turns the water inside the tubes into hot, dry steam. Currently, the steam is at a temperature of roughly 1000 degrees Fahrenheit. The next phase is the turbine, where thermal energy from this process is converted to mechanical energy.
 Turbine / Generator
Turbine / Generator
The first of a sequence of turbines receives high pressure steam that is now flowing from the boiler at 3500 pounds per square inch and 1000 degrees Fahrenheit. The steam then loops back to the furnace before continuing to the second turbine as it expands between layers of turbine blades positioned on the turbine shaft. The final machine in the chain, the generator, receives power from the steam, which rotates the series of turbines at a rate of 3,600 revolutions per minute.
An electrical charge of 34,481 amps at 18,000 volts is continuously produced by the generator. The electricity then leaves the facility and starts its journey to customers from this point.
The steam is discharged from the turbine after it has been utilised to generate power and is then transferred to a condenser to be converted back into water. Steam moves over pipes filled with chilled water from the cooling towers inside the condenser. The steam returns to the boilers after condensing to water in order to restart the steam generation process.
Water Reuse and Condenser
 The chilled water in the condenser pipes warms up as the steam condenses, so it is sent to a cooling tower. These towers, which have no mechanical parts, are known as natural draught cooling towers. Small droplets of water are created inside because of the water splashing over a number of baffles. These droplets interacted with air from the tower’s open bottom, causing some of the water to evaporate and the remaining water to cool by as much as 27 degrees. Returning to the condenser to cool more steam once again, the newly chilled water.
The chilled water in the condenser pipes warms up as the steam condenses, so it is sent to a cooling tower. These towers, which have no mechanical parts, are known as natural draught cooling towers. Small droplets of water are created inside because of the water splashing over a number of baffles. These droplets interacted with air from the tower’s open bottom, causing some of the water to evaporate and the remaining water to cool by as much as 27 degrees. Returning to the condenser to cool more steam once again, the newly chilled water.
 Scrubbers
Scrubbers
First Energy has invested more than $5 billion in environmental preservation since 1971, and our plants include air quality control systems to get rid of fly ash and sulphur dioxide.
Fly ash is eliminated mechanically, and sulphur dioxide is eliminated chemically using lime. These operations take place in sizable ductwork scrubber trains that are situated between the boiler and the chimney of the unit. Boiler gases are sprayed with slurry, a solution of water and lime, as they pass through scrubbing vessels.
The slurry absorbs the contaminants, including the sulphur dioxide particles, which then settle to the bottom of the vessels. The clean gases are expelled through the chimney by a fan.
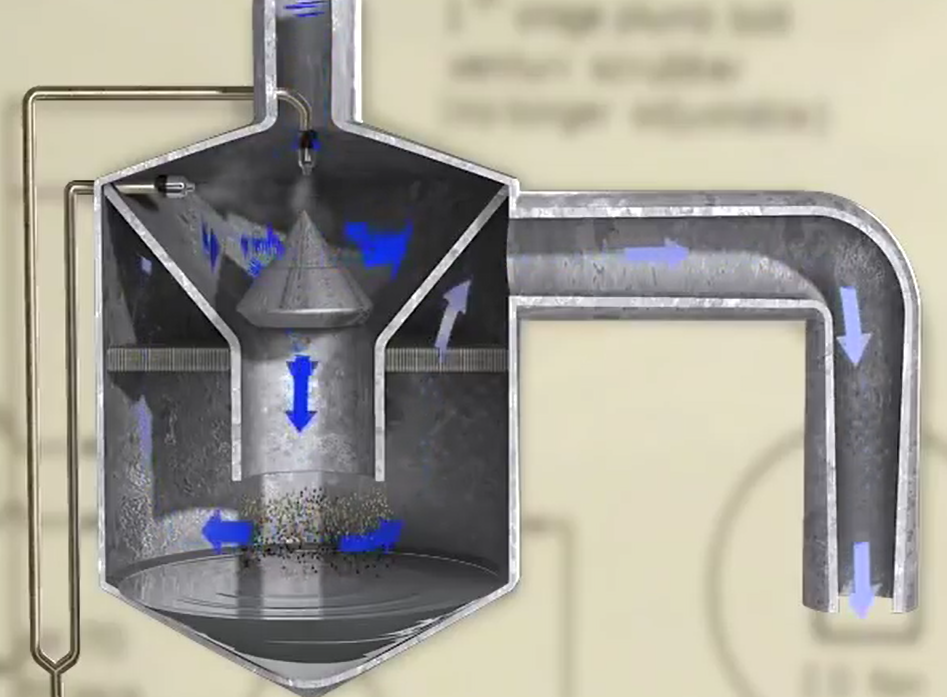 By the time this occurs, 92 percent of sulphur dioxide and more than 99 percent of particles have been eliminated. A facility like this one has the capacity to remove more than 400,000 tonnes of sulphur dioxide annually.
By the time this occurs, 92 percent of sulphur dioxide and more than 99 percent of particles have been eliminated. A facility like this one has the capacity to remove more than 400,000 tonnes of sulphur dioxide annually.
Gypsum Process
The scrubbing procedure produces enormous volumes of calcium sulphite, a by-product. A daily production of more than 3 million gallons of calcium sulphites slurry might result from this concept. First Energy developed a method to transform that waste product into gypsum, a useful building resource that is used to create wallboard or drywall.
The scrubber slurry is thickened and pumped through a forced oxidation system using gypsum or fog. Gypsum is created by adding oxygen after which it is dried, processed, and transported to a maker of wallboard. Gypsum is recycled by First Energy on a yearly basis in amounts equivalent to producing wallboard for 70,000 new homes.
Fly Ash Recycling
Another way to recover coal ash, also known as fly ash, that can be recycled or dumped in landfills, is via precipitators. Fly ash from the combustion process is removed mechanically in this method. Fly ash from a vibrating wire is shaken by these poles, also known as wrappers. Fly ash-containing gas from the furnace enters the box. Fly ash falls into a chamber for storage before being blown into silos and loaded into a truck. Several items, including concrete grout, roofing shingles, granules, and anti-skid road materials, are made with fly ash from first energy plants.
 SCR System
SCR System
In order to lower nitrogen oxide emissions, First Energy also employs selective catalytic reduction technology, or SCR. SCR systems function similarly to a car’s catalytic converter. Ammonia is combined with flue gas that contains nitrogen oxide emissions from the combustion process.
 The nitrogen oxides and ammonia react as a result of the combined gases passing through several catalytic layers. The reaction transforms the nitrogen oxides into pure nitrogen and water vapour, two safe chemicals that account for 80% of the air we breathe. Through the station stacks, both substances are reintroduced to their natural surroundings.
The nitrogen oxides and ammonia react as a result of the combined gases passing through several catalytic layers. The reaction transforms the nitrogen oxides into pure nitrogen and water vapour, two safe chemicals that account for 80% of the air we breathe. Through the station stacks, both substances are reintroduced to their natural surroundings.
Electricity’s Path
Let’s examine how power is brought to our buildings and places of business. Just outside the plant, transformers increase the power’s voltage from 18,000 volts to 345,000 volts so that it may be sent across vast distances via transmission lines. In order to move the voltage via distribution lines connecting to the utility poles, substations situated along the route lower the voltage. Transformers mounted on poles scaled down the power as it approached client locations for usage in residences and commercial buildings.
- Coal based power plants
How does a thermal power plant work use coal
Nearly half of the world’s energy demand is met by thermal power plants, which use water as their working fluid. Today’s thermal power plants can operate with high levels of efficiency by adhering to strict environmental regulations. This section will provide a step-by-step breakdown of how a coal-based thermal power plant does this.
 Generators and Turbine
Generators and Turbine
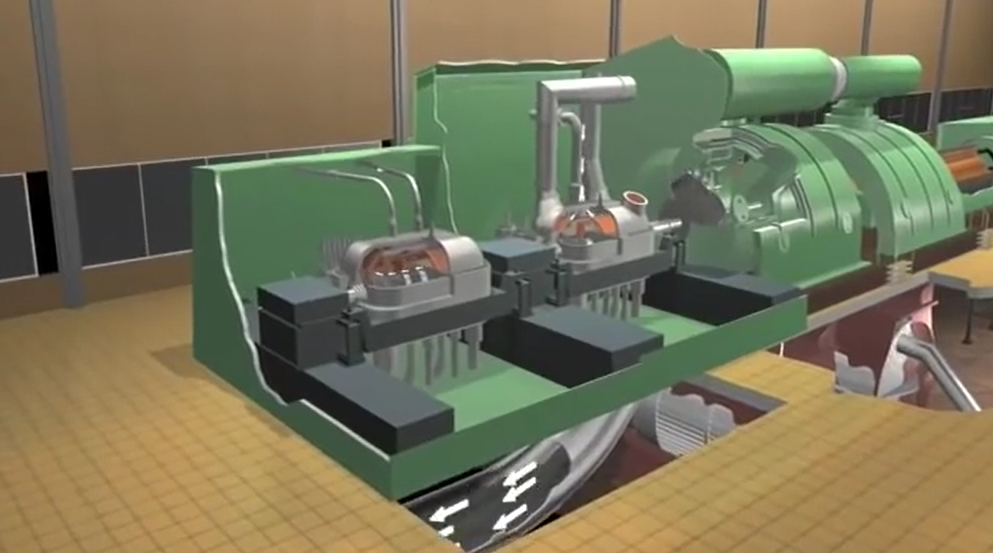
We will be able to produce electricity by turning the generator’s shaft. The steam turbine at the centre of the power plant drives the generator. High pressure, high temperature steam must be injected into the steam turbine’s inlet in order for it to turn. The pressure and temperature of the turbine decrease as it draws energy from the high-energy fluid toward the outlet. You can get a better look at the steam turbine’s unusually formed rotor blades.
 In high-capacity power plants, various stages of steam turbines, like
In high-capacity power plants, various stages of steam turbines, like
- turbine with high pressure
- turbine with intermediate pressure
- turbines with little pressure.
As a result, we have achieved our goal and the generator is now producing electricity. We can repeat the process if we can return the low pressure and low temperature steam to their initial states, which were of a much higher pressure and temperature.
Increasing pressure is the first stage. For this, a compressor can be used. However, because compressing steam requires a significant amount of energy, such a power plant will not operate at all efficiently. The simple solution is to increase pressure while turning the steam into liquid.
Condenser
We’ll introduce condenser heat exchangers, which are located below the low-pressure turbine, for this function.
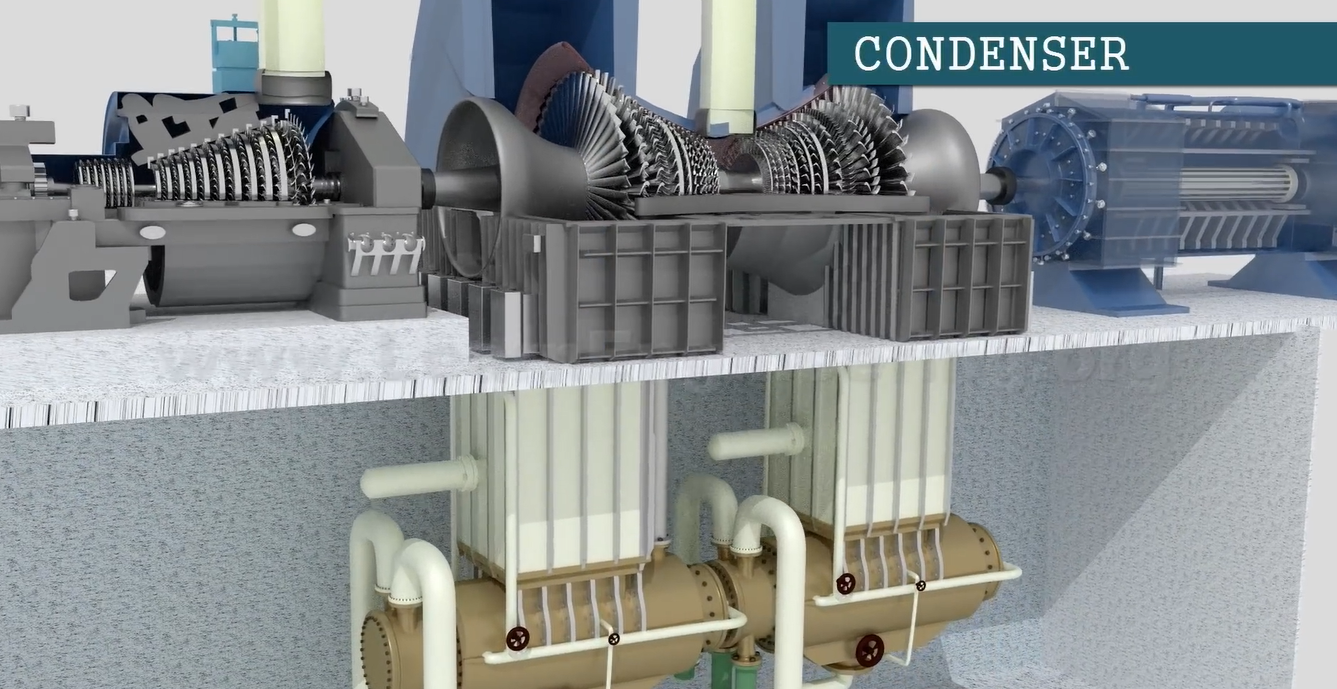 A stream of cold water is running through the tubes in the condenser. Condensation results from the steam rejecting heat to this liquid stream. We can now use a pump to raise the feedwater’s pressure.
A stream of cold water is running through the tubes in the condenser. Condensation results from the steam rejecting heat to this liquid stream. We can now use a pump to raise the feedwater’s pressure.
Feed water Pump
Multistage centrifugal pumping is frequently employed for this purpose. In this manner, the pressure will return to its initial condition. The next step is to reset the temperature to its initial setting. In order to achieve this, a boiler is used to add heat to the pump’s outflow. Typically, water tube boilers are the type of boiler used in high-capacity power plants.
Boiler
That bird in the boiler is pulverised coal. The entering water first travels through an economizer session where it will absorb energy from the flue gas before flowing via a downcomer and onto water walls, where it will eventually turn into steam. At a steam drum, the pure steam is separated. The working fluid has returned to its initial high pressure and high temperature state.
Continual power generation is possible by feeding this steam back into the steam turbine on a continuous basis. However, a power plant operating on this fundamental ranking cycle will have a very poor efficiency and a low capacity. With the aid of a few straightforward procedures, we may greatly improve the performance of the power plant.
Superheating
Even after the liquid has been transformed into steam in the instance of superheating, further heat is supplied, causing the steam to become superheated. The cycle is more effective at higher steam temperatures. Just keep in mind the maximum thermal efficiency theory, Carnot’s.
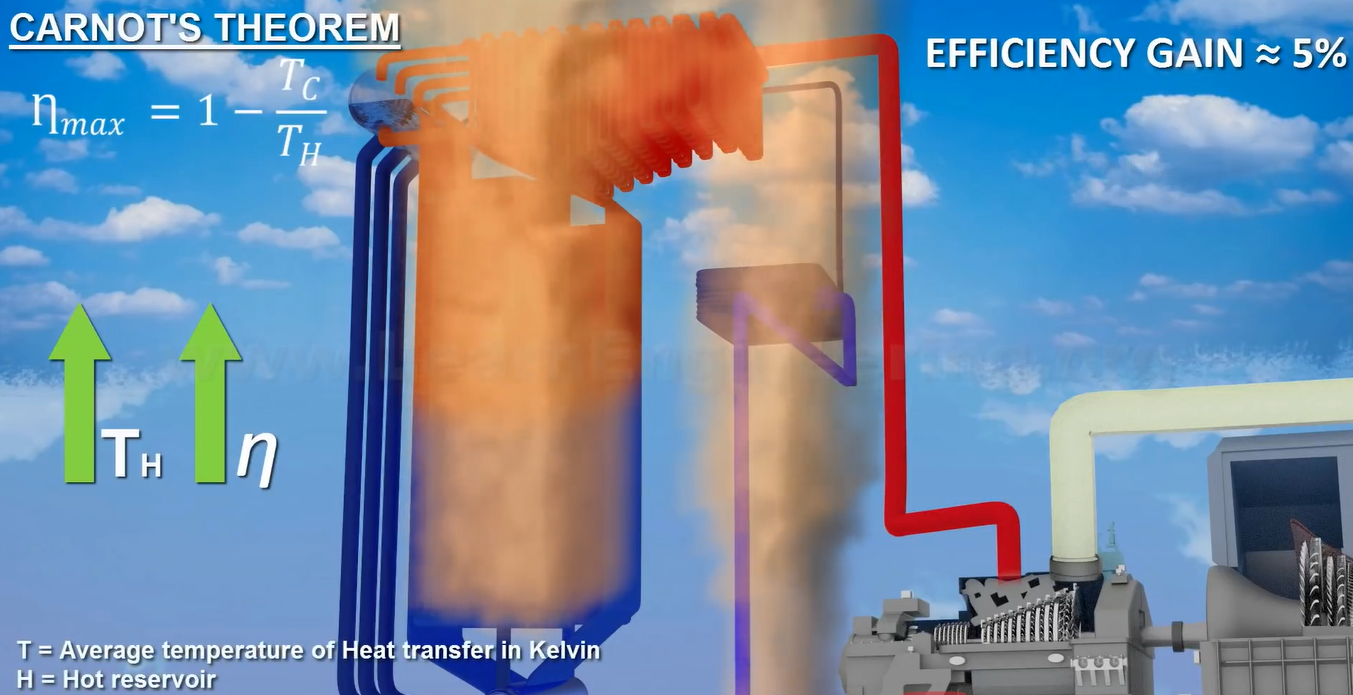 However, materials used in steam turbines cannot tolerate temperatures higher than 600 degrees Celsius. So, superheating is only possible at that level.
However, materials used in steam turbines cannot tolerate temperatures higher than 600 degrees Celsius. So, superheating is only possible at that level.
Reheating stage
As the steam moves along the blade’s rows, its temperature drops. Therefore, adding more heat after the first turbine stage is a wonderful approach to boost the power plant’s efficiency. Reheating causes the temperature of the steam to rise once more, producing a high-power output and improving efficiency.
Despite complex sealing procedures, the power plant’s low-pressure sides are prone to sucking in ambient air. Over time, the boiler material will degrade due to the dissolved gases in the feedwater. An open feedwater heater is used to remove these dissolved gases. In order for the feedwater steam bubbles to effectively absorb the dissolved gases, hot steam from the turbine is blended into them.
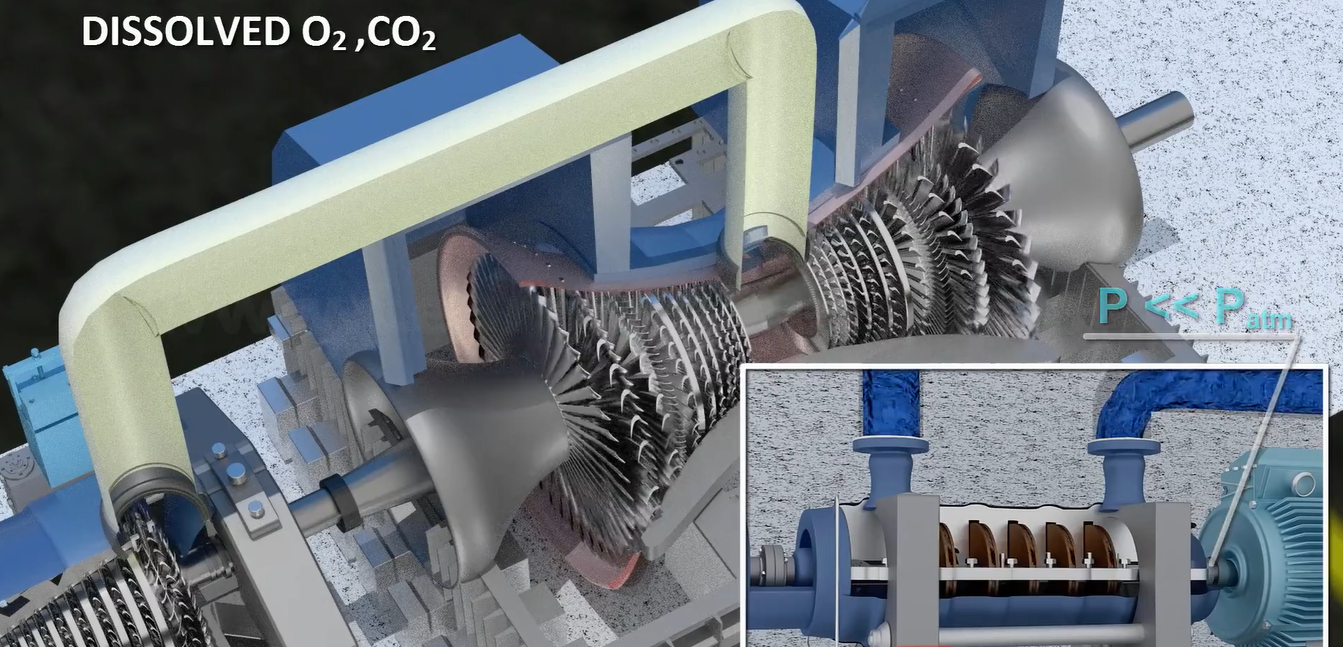
Additionally, the mixing warms up the feed water, further enhancing the power plant’s efficiency. These methods enable the modern power plant to operate at an efficiency level between 40 and 45 percent. We’ll now examine how heat rejection and addition are carried out in a real power plant. A cooling tower is used to supply the cooled liquid to the condenser.
Cooling tower
The condenser outlet sprays hot water into the cooling tower, creating a natural air draught that causes the water to lose heat. This is how the condenser intake is consistently supplied with a colder liquid. Many pollutants are produced by the burning of coal on the heat addition side. These contaminants cannot be released into the atmosphere directly. Therefore, the exhaust gases are cleaned in an electrostatic precipitator before being transferred to a stack. To capture the polluting particles, the ESP employs high voltage static electricity on plates. We trust that you now have a better understanding of how thermal power plants operate.
Frequently Asked Questions
1) How does coal pollution impact the environment?
Burning coal discharges hazardous and cancer-causing compounds into our air, water, and land in addition to increasing greenhouse gas pollution, which has a negative impact on the health of miners, employees, and the communities around them.
2) How can we reduce the environmental impact of coal use?
Optimizing current facilities to produce more energy using the same amount of coal while reducing emissions. developing and improving the greatest combustion technology now accessible, such as circulating fluidized-bed (CFB) technology, which includes supercritical and ultra-supercritical combustion.
3) How does coal mining affect the atmosphere?
Methane emissions from the mines, which contribute to global warming pollution, and particle matter (PM) emissions, which can result in substantial lung damage and even early mortality, are the two main causes of air pollution throughout the coal producing process.
4) How long will coal last?
The recoverable coal reserves would last about 435 years, while the recoverable reserves in producing mines would last about 21 years, based on the estimated 0.577 billion short tons of coal output in the United States in 2021. Depending on changes in production and reserve projections, the actual number of years those reserves last will vary.
5) How does coal mining affect plants?
The disruption of vegetation brought on by coal mining results in biomass loss and reduces the capacity of vegetation to absorb atmospheric CO2. The amount of vegetation biomass lost because of coal mining between 2001 and 2010 was 2,608,48 t, with a loss rate of 33.48 gC/m2 per year.
6) How does coal affect human health?
They comprise a number of different heavy metals as well as mercury, lead, sulfur dioxide, nitrogen oxides, and particulates. The negative effects on health might include everything from asthma and respiratory issues to brain damage, heart issues, cancer, neurological diseases, and early death.
7) What are coals characteristics?
Inorganic elements are held molecularly by the organic stuff in coal as well as isolated minerals, water, and gases that are confined in microscopic pores. Coal is largely composed of carbon, hydrogen, and oxygen on an organic level, with traces of sulfur and nitrogen.
8) What element is important in the production of coal?
With numerous other trace atoms, carbon makes up the majority of its makeup. Because of its abundant natural reserves and high energy density, coal may be used to generate electricity in coal-fired power plants as well as for heating in some locations.
9) What are the 4 main requirements for the formation of coal?
Coal is formed in four stages: peat, lignite, bituminous coal, and anthracite coal. The stage is determined by the circumstances under which the plant remains were placed after being buried; the higher the pressure and heat, the higher the rank of coal.