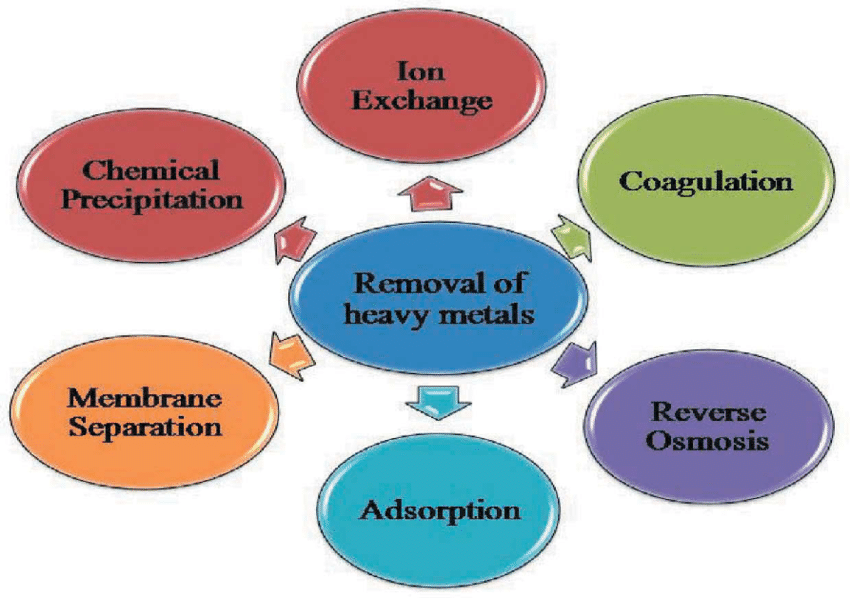
Various Technologies and Processes Used to Remove Metal Content from Kraft Lignin
Introduction:
Kraft lignin is a byproduct of the pulp and paper industry, and it contains various impurities, including metal ions and organic contaminants. To remove metal content from kraft lignin, various technologies and processes can be employed. The choice of method depends on the specific requirements and the nature of the impurities present. Here are some common technologies and processes used for metal removal from kraft lignin:

- Precipitation:
This method involves adjusting the pH of the kraft lignin solution to cause the metal ions to form insoluble precipitates. Common chemicals used for precipitation include lime (calcium hydroxide), aluminum sulfate, and ferric chloride. The precipitates can then be separated from the lignin solution through filtration or sedimentation. - Ion Exchange:
Ion exchange resins can be used to selectively remove metal ions from kraft lignin solutions. These resins contain specific functional groups that exchange metal ions in solution for ions on the resin. The resin can be regenerated for reuse by eluting the metal ions with a suitable solution. - Adsorption:
Adsorption processes involve using adsorbents such as activated carbon or silica gel to trap metal ions from the lignin solution. This is an effective method for removing organic contaminants along with metal ions. - Chelation:
Chelating agents like ethylenediaminetetraacetic acid (EDTA) or diethylenetriaminepentaacetic acid (DTPA) can be added to the lignin solution. These chelating agents form stable complexes with metal ions, making them easier to remove through precipitation or other separation methods. - Solvent Extraction:
Organic solvents can be used to extract metal ions from the lignin solution. This process relies on the differential solubility of metal complexes in various solvents. After extraction, the metal-rich solvent can be separated from the lignin solution. - Electrochemical Methods:
Electrochemical techniques like electrocoagulation and electro flotation can be employed to remove metal ions from kraft lignin. In these methods, an electric current is applied to induce the coagulation or flocculation of metal ions, which can then be easily separated. - Ultrafiltration and Membrane Processes:
Membrane filtration methods, such as ultrafiltration, nanofiltration, or reverse osmosis, can be used to selectively separate metal ions and other impurities based on their molecular size and charge. - Biological Treatment:
Biological processes, such as bioleaching and biosorption, involve the use of microorganisms or biomass to selectively adsorb or metabolize metal ions from the lignin solution.The choice of the most appropriate method depends on factors like the type and concentration of metal ions, the desired purity of the kraft lignin, and economic considerations. Often, a combination of these methods may be used in a multi-step process to achieve the desired level of metal removal from kraft lignin.
Metal Content in Kraft Lignin
The achievable metal content in kraft lignin produced using current production technology can vary depending on several factors, including the specific process conditions and the efficiency of metal removal techniques employed.
In many industrial processes, the metal content in kraft lignin is typically reduced to relatively low levels to meet quality and purity requirements for various applications. Achieving a metal content of 500 ppm (parts per million) is relatively common with current production technology. However, the ability to reduce it to below 20 ppm would depend on several factors:
- Process Optimization:
The metal content in kraft lignin can be reduced through process optimization, including adjusting the pulping and recovery processes, as well as improving washing and separation steps to minimize metal contamination. - Metal Removal Techniques:
The choice and effectiveness of metal removal techniques, such as precipitation, ion exchange, adsorption, or other methods mentioned earlier, play a crucial role in achieving lower metal content levels. - Quality Requirements:
The desired metal content may also be influenced by the specific application of the kraft lignin. Some applications may require very low metal content, while others may tolerate higher levels. - Economic Considerations:
Achieving extremely low metal content levels can be costly, so economic factors may also play a role in determining the target metal content.
It’s certainly possible to reduce the metal content in kraft lignin to below 20 ppm with appropriate processes and technologies, but it may require significant investments in equipment and process modifications. The exact achievable metal content will vary from one production facility to another and depend on the specific circumstances and goals of the operation. Companies and researchers in the pulp and paper industry are continually working on improving lignin purification processes to meet the requirements of various applications.
Yield of Metal Content from Kraft Lignin
The maximum yield of metal content from kraft lignin typically involves minimizing the metal content in the recovered lignin while maximizing the yield of lignin itself. Different opinions and strategies can be found among researchers and industry experts, and these opinions may vary based on factors like the specific application of the lignin and the available resources. Here are some additional considerations and opinions on achieving the maximum yield of metal contents from kraft lignin:
- Selecting the Right Wood Source:
The type of wood used in the pulping process can affect the metal content in kraft lignin. Some wood species naturally contain higher levels of metals, so selecting wood with lower metal concentrations can be a strategy to start with lower levels of metal in the lignin. - Pulping Process Optimization:
Optimizing the kraft pulping process itself can reduce the metal content in the resulting lignin. Properly controlled cooking conditions, such as temperature, time, and chemical additives, can influence metal leaching and retention. - Recovery Boiler and Black Liquor Management:
The efficiency of the recovery boiler and the management of black liquor, a byproduct of the kraft pulping process, are critical. Proper operation of the recovery boiler can minimize metal carryover into the recovery cycle and, consequently, into the lignin. - Cleaning and Washing Steps:
Effective washing and cleaning steps during the pulping and lignin extraction process can significantly reduce metal impurities. Improved washing and separation technologies can help achieve higher yields of purified lignin - Advanced Separation Techniques:
Employing advanced separation and purification techniques, such as membrane processes, can contribute to achieving high-purity lignin with lower metal content. - Quality Control and Monitoring:
Continuous monitoring and quality control measures can help identify and address issues related to metal contamination in the lignin production process.
- Integration with Other Processes:
Some facilities integrate lignin recovery with other industrial processes, such as biorefineries or wastewater treatment, to further reduce metal content and improve lignin purity. - Research and Innovation:
Ongoing research and development efforts in the pulp and paper industry are aimed at developing more efficient and cost-effective methods for lignin purification and metal removal.
Ultimately, achieving the maximum yield of metal content from kraft lignin involves a combination of process optimization, technology selection, and quality control measures. It’s essential to balance the cost of implementing these measures with the specific requirements of the end-use application for the lignin. Different facilities and applications may prioritize different strategies based on their unique circumstances and goals.
3 Techniques and Processes to Remove the Metal Content from Kraft Lignin.
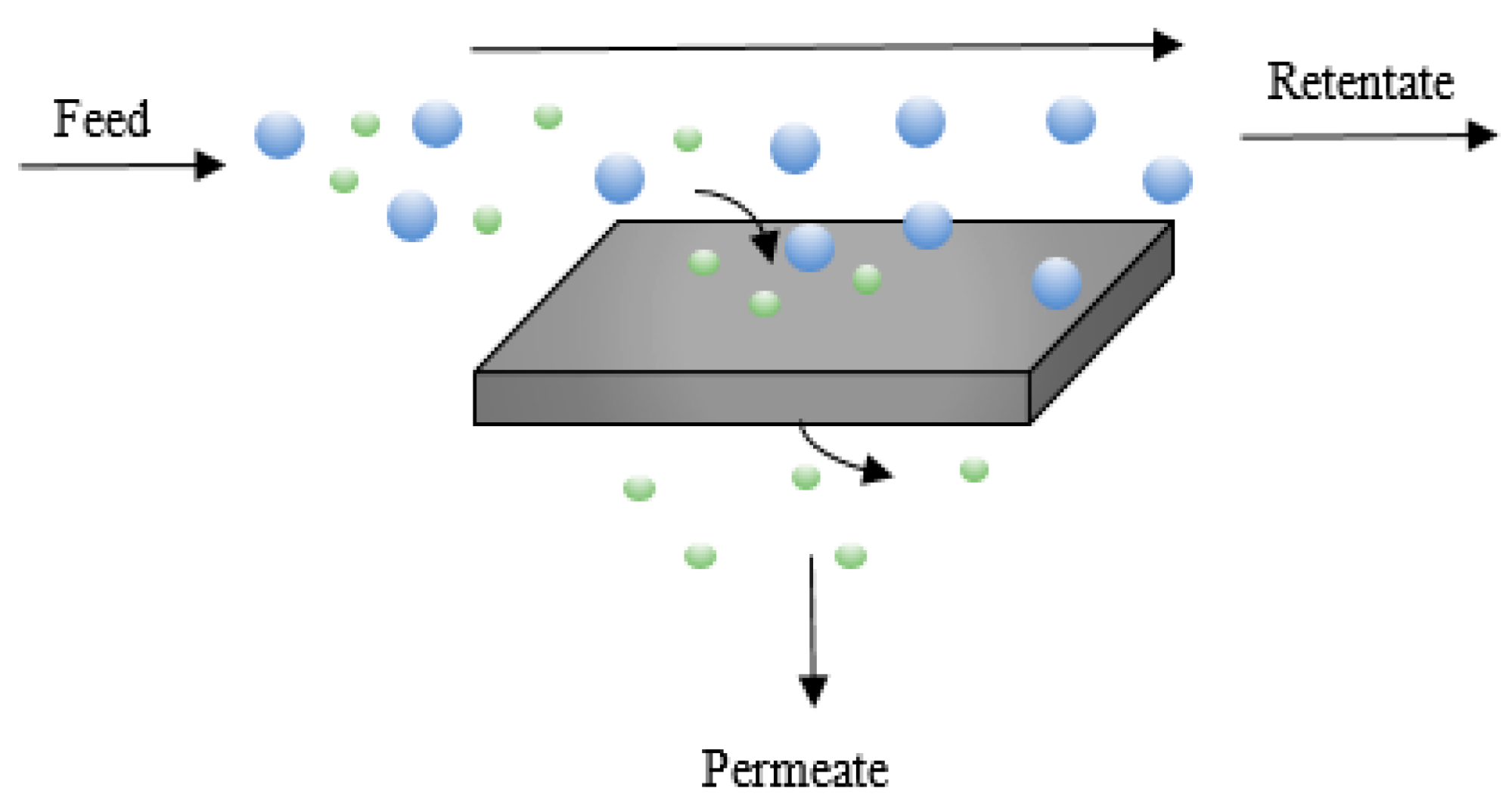
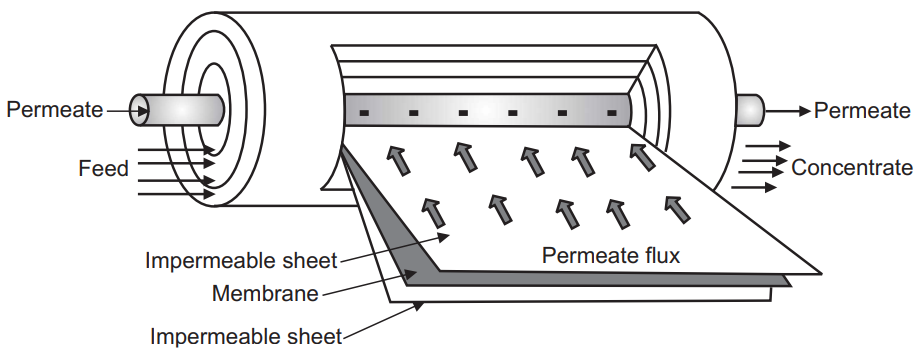
Ultrafiltration and Membrane Processes
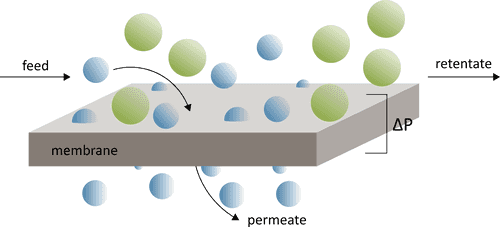
Ultrafiltration and other membrane processes are effective and widely used techniques for the separation and purification of various materials, including removing metal contents from kraft lignin. These processes rely on semi-permeable membranes to separate components based on their molecular size, shape, and charge. In the context of kraft lignin purification, ultrafiltration and membrane processes offer several advantages, including scalability, energy efficiency, and the ability to achieve high levels of metal removal without the need for chemical additives. In this comprehensive discussion, we will explore the principles, applications, advantages, challenges, and recent advancements in ultrafiltration and membrane processes for metal removal from kraft lignin.
- Principles of Ultrafiltration and Membrane Processes:
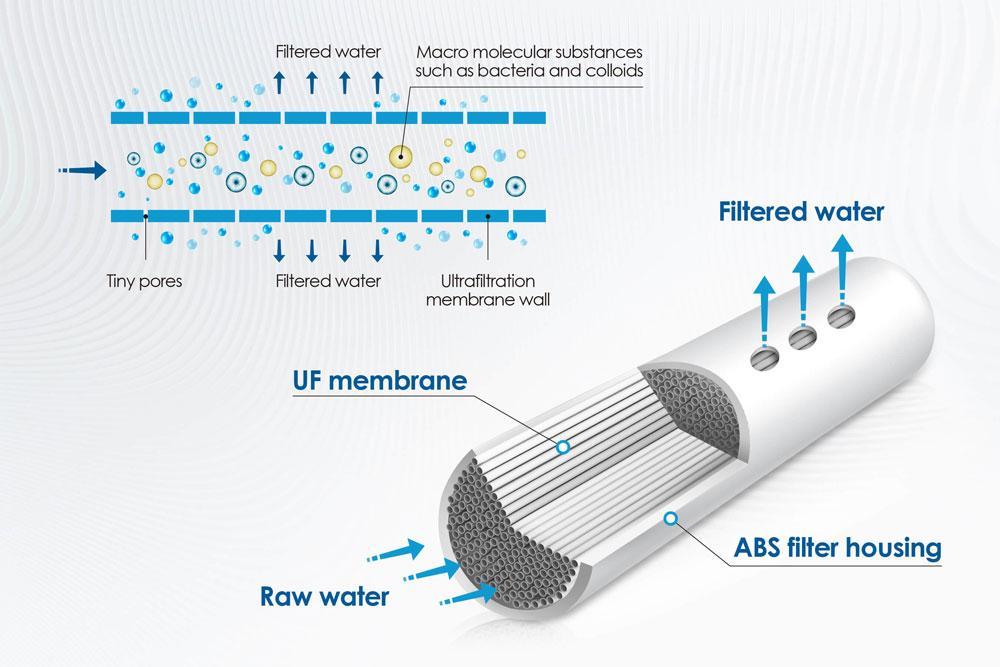
Ultrafiltration and membrane processes are based on the principles of size exclusion and sieving. They involve the use of semi-permeable membranes with defined pore sizes that allow the passage of water and smaller solute molecules while retaining larger molecules, particles, and ions. The separation is primarily driven by pressure differentials across the membrane.In the context of metal removal from kraft lignin, the following principles are essential:- Pore Size Selection:
The selection of an appropriate membrane with a specific pore size is crucial. Ultrafiltration membranes typically have pore sizes in the range of 1 to 100 nanometres (nm). This allows them to retain metal ions and larger organic molecules while permitting water and smaller solutes to pass through.
- Transmembrane Pressure (TMP):
The application of pressure on the feed side of the membrane, known as the transmembrane pressure, is used to force the liquid through the membrane. The pressure gradient facilitates the separation process. - Size-Based Separation:
The smaller metal ions and other impurities are rejected by the membrane, while the larger kraft lignin molecules pass through. This size-based separation enables the removal of metal content from the lignin solution.
- Pore Size Selection:
- Applications of Ultrafiltration and Membrane Processes in Kraft Lignin Purification:
Ultrafiltration and membrane processes have found several applications in the pulp and paper industry for the purification of kraft lignin. Some key applications include:- Lignin Recovery:
Ultrafiltration can be used as a part of the lignin recovery process to separate kraft lignin from black liquor, a byproduct of the kraft pulping process. By removing metal ions and other impurities, the recovered lignin can be of higher quality and suitable for various applications.
- Pretreatment
Ultrafiltration can serve as a pretreatment step before other downstream processes, such as precipitation or ion exchange. By reducing the metal content in the lignin solution, these subsequent processes can be more efficient and cost-effective. - Purification:
Ultrafiltration is employed to purify lignin solutions obtained from different sources. It helps in achieving high lignin purity by removing metal ions, ash, and other contaminants.
- Concentration:
In some cases, ultrafiltration is used to concentrate kraft lignin solutions. By removing water through the membrane, the lignin content can be increased, making it suitable for specific applications.
- Lignin Recovery:
- Working Mechanism of Ultrafiltration and Membrane Processes:
Ultrafiltration and membrane processes work based on the principle of selectively allowing certain substances to pass through a semi-permeable membrane while retaining others. These processes are commonly used for separation, purification, and concentration of various materials, including removing metal content from solutions like kraft lignin. Here’s an overview of the working mechanism of ultrafiltration and membrane processes:-
- Membrane Structure:
The heart of ultrafiltration and membrane processes is the semi-permeable membrane. These membranes are typically made from various materials, including polymers, ceramics, or metals. The structure of the membrane is crucial and consists of pores or channels that determine what can pass through and what is retained.
- Size Exclusion:
The key principle behind ultrafiltration is size exclusion. The membrane has specific pore sizes, usually in the range of 1 to 100 nanometers (nm). These pores are smaller than the particles or solutes in the feed solution but large enough to allow the passage of solvent molecules (usually water). - Pressure Differential:
A pressure differential, known as the transmembrane pressure (TMP), is applied across the membrane. This pressure pushes the liquid (usually the feed solution) through the membrane.
- Separation Process:
The process occurs as follows:-
- Permeate:
The substances that are smaller than the pore size of the membrane pass through the pores and become part of the permeate. In the context of kraft lignin purification, this would include water and smaller molecules.
- Retentate:
Substances that are larger than the pore size are unable to pass through the membrane and are retained on the feed side. This includes larger molecules, particles, and metal ions.
- Permeate:
-
- Selectivity:
The selectivity of the membrane is crucial. In the case of kraft lignin purification, the membrane should allow lignin molecules to pass while retaining metal ions and other impurities. This selectivity is determined by the membrane’s pore size and surface properties.
- Concentration and Separation:
In addition to separating components based on size, membrane processes can also be used to concentrate or dilute solutions. By applying the appropriate pressure, you can increase the concentration of solutes in the retentate or reduce them in the permeate.
- Crossflow Filtration:
In many ultrafiltration and membrane processes, crossflow filtration is employed. In crossflow filtration, the feed solution flows parallel to the membrane surface. This continuous flow helps reduce fouling (the accumulation of particles or impurities on the membrane surface) by constantly sweeping away retained materials.
- Cleaning and Maintenance:
Membrane systems require regular cleaning and maintenance to ensure optimal performance. Fouling, scaling, or other issues may necessitate membrane replacement or cleaning with appropriate solutions.
- Membrane Structure:
-
- Types of Membranes:
Different types of membranes are used depending on the specific application. For example, inorganic ceramic membranes are known for their chemical resistance and are used in harsh environments, while polymeric membranes are widely used for their flexibility and cost-effectiveness.
- Applications:
Ultrafiltration and membrane processes are used in a wide range of industries, including food and beverage processing, wastewater treatment, pharmaceuticals, and, as discussed earlier, in the pulp and paper industry for kraft lignin purification.
- Types of Membranes:
In summary, ultrafiltration and membrane processes work by utilizing semi-permeable membranes with specific pore sizes to selectively separate substances based on their size and, in some cases, their charge and shape. These processes are valuable tools for various applications where the purification, concentration, or separation of specific components from a solution is required, including the removal of metal contents from kraft lignin solutions.
-
- Advantages of Ultrafiltration and Membrane Processes:
Ultrafiltration and membrane processes offer several advantages for removing metal contents from kraft lignin:-
- Selective Removal:
Membrane processes are highly selective and can remove metal ions and impurities based on their size, allowing kraft lignin molecules to pass through without significant loss.
- Minimal Chemical Usage:
Unlike some other purification methods, ultrafiltration and membrane processes do not require the addition of chemicals, making them environmentally friendly and cost-effective. - Energy Efficiency:
These processes are energy-efficient and can be operated at relatively low pressures compared to processes like reverse osmosis.
- Scalability:
Ultrafiltration and membrane systems can be easily scaled up to accommodate different production volumes, making them suitable for industrial applications.
- Continuous Operation:
Membrane processes can be operated continuously, leading to consistent and efficient metal removal from kraft lignin solutions.
- Reduced Environmental Impact:
By reducing the need for chemical additives and producing high-purity lignin, these processes contribute to a reduced environmental impact of the lignin production process.
- Selective Removal:
-
- Challenges and Considerations:
While ultrafiltration and membrane processes offer many advantages, they also come with specific challenges and considerations in the context of removing metal contents from kraft lignin:- Membrane Fouling:
One of the primary challenges is membrane fouling, where particles and impurities can accumulate on the membrane surface, reducing its efficiency. Strategies to mitigate fouling include regular cleaning, using anti-fouling membranes, and optimizing operating conditions.
- Pore Size Selection:
Selecting the appropriate membrane with the right pore size is critical. If the pore size is too large, some metal ions may pass through, while if it’s too small, lignin molecules may be rejected. - Operating Conditions:
The choice of operating conditions, including transmembrane pressure and temperature, can impact the performance and efficiency of the process.
- Maintenance:
Membrane systems require regular maintenance to ensure optimal performance. This includes membrane replacement or cleaning.
- Cost Considerations:
While ultrafiltration and membrane processes can be cost-effective in the long run, the initial capital investment for membrane systems can be significant.
- Membrane Fouling:
- Recent Advancements and Research
Researchers and industry experts are continually working on advancements in ultrafiltration and membrane processes for metal removal from kraft lignin. Some recent developments and research areas include:-
- Nanofiltration Membranes:
The development of nanofiltration membranes with precise pore sizes allows for even greater control over metal removal, potentially achieving lower metal content in purified lignin.
- Anti-Fouling Coatings:
The use of anti-fouling coatings on membranes helps reduce fouling and extend membrane life, leading to more efficient operations. - Hybrid Processes:
Combining ultrafiltration or membrane processes with other separation techniques, such as adsorption or precipitation, can enhance metal removal efficiency.
- Process Integration:
Researchers are exploring ways to integrate ultrafiltration and membrane processes into existing kraft lignin production processes for seamless metal removal.
- Environmental Impact Assessment:
Recent studies have focused on assessing the environmental impact of membrane processes and optimizing them for sustainability.
- Energy Efficiency:
Efforts are ongoing to further improve the energy efficiency of membrane systems through the development of low-energy membrane materials and designs.
- Nanofiltration Membranes:
Ultrafiltration and membrane processes are effective and versatile techniques for removing metal contents from kraft lignin. They offer several advantages, including selectivity, minimal chemical usage, energy efficiency, and scalability. However, challenges such as membrane fouling, and the selection of appropriate operating conditions must be addressed to ensure optimal performance. Recent advancements in membrane technology, anti-fouling coatings, and process integration have the potential to further improve the efficiency of metal removal from kraft lignin. As the demand for high-quality lignin continues to grow in various industrial applications, ultrafiltration and membrane processes are likely to play a significant role in enhancing the purity and utility of kraft lignin.
-
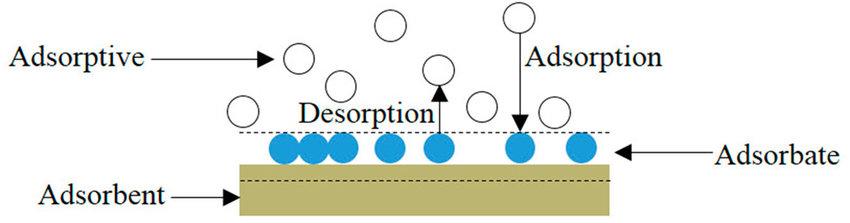
Absorption Processes for Removing Metal Contents from Kraft Lignin
Absorption processes are widely employed in various industries to remove metal ions and other impurities from solutions. In the context of kraft lignin purification, absorption processes offer an effective means to selectively remove metal content, resulting in higher purity lignin suitable for a range of applications. This comprehensive discussion will delve into the principles, mechanisms, applications, advantages, challenges, and recent advancements in absorption processes for metal removal from kraft lignin.
//image
- Principles of Absorption:
Absorption is a separation process that relies on the differential solubility of substances in a liquid or gas phase (the absorbent) as compared to their solubility in another liquid or gas phase (the feed solution or gas stream). The key principles of absorption processes are as follows:- Contact between Phases:
The feed solution containing the target metal ions and an absorbent are brought into contact with each other. The metal ions are transferred from the feed solution to the absorbent phase.
- Differential Solubility:
Metal ions in the feed solution have a higher affinity for the absorbent phase than for the original solution. As a result, they dissolve or migrate into the absorbent. - Mass Transfer:
The absorption process involves mass transfer, where the metal ions move from the feed solution phase to the absorbent phase. This mass transfer occurs until equilibrium is reached.
- Equilibrium and Saturation:
Equilibrium is attained when the concentration of metal ions in the absorbent phase reaches a maximum and no further mass transfer occurs. The absorbent phase is then considered “saturated” with the metal ions. - Desorption (Optional):
In some cases, the metal ions can be desorbed or removed from the saturated absorbent, typically through a different solvent or by changing the conditions. This step may be necessary if metal recovery is required.
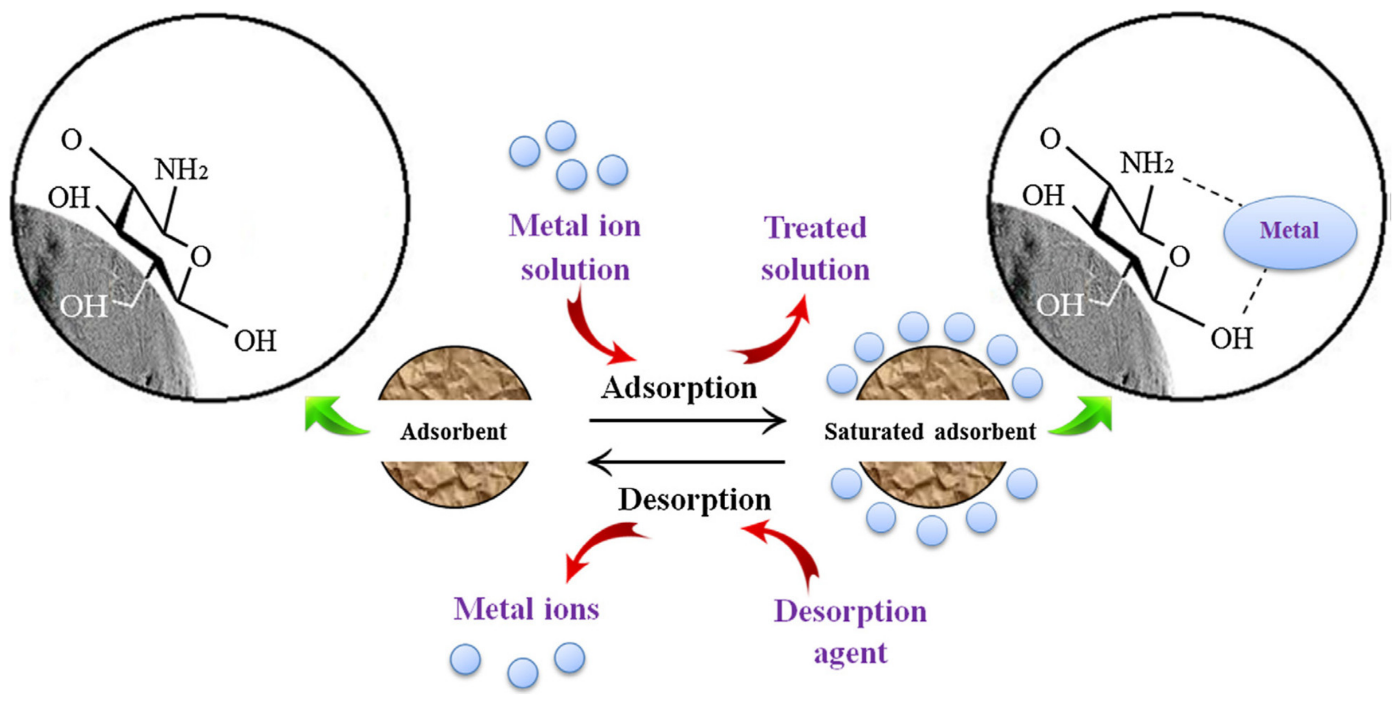
- Contact between Phases:
- Applications of Absorption in Kraft Lignin Purification:
Absorption processes find several vital applications in the removal of metal contents from kraft lignin:-
- Lignin Recovery:
Absorption can be used as a method to recover and purify lignin from black liquor, a byproduct of the kraft pulping process. Removing metal ions is essential to ensure the quality of the recovered lignin.
- Pretreatment
Ultrafiltration can serve as a pretreatment step before other downstream processes, such as precipitation or ion exchange. By reducing the metal content in the lignin solution, these subsequent processes can be more efficient and cost-effective. - Purification:
Absorption is employed to purify kraft lignin solutions obtained from various sources. It helps in achieving high lignin purity by selectively removing metal ions and other impurities.
- Pre-treatment:
Absorption can serve as a pre-treatment step before other downstream processes, such as membrane filtration or precipitation. By reducing the metal content in the lignin solution, these subsequent processes can be more efficient and cost-effective.
- Concentration:
In some cases, absorption can be used to concentrate kraft lignin solutions. By removing water through the absorbent phase, the lignin content can be increased, making it suitable for specific applications.
- Lignin Recovery:
-
- Working Mechanism of Adsorption process
The absorption process is a separation technique that involves the transfer of one or more components (usually gases or solutes) from a gas or liquid phase into another phase (typically a liquid or solid absorbent). The process relies on differences in the solubility and affinity of the components between the two phases. Here’s a detailed explanation of the working mechanism of the absorption process:-
- Contact between Phases:
The absorption process begins by bringing the gas or liquid mixture containing the component to be removed (known as the solute or absorbate) into contact with the absorbent phase. The absorbent is usually a liquid or solid material chosen for its ability to selectively dissolve or capture the solute.
- Mass Transfer:
When the two phases come into contact, the solute molecules start to migrate from the gas or liquid phase into the absorbent phase. This migration is driven by differences in chemical potential, which are influenced by factors such as concentration, temperature, and pressure. Mass transfer occurs until equilibrium is reached, meaning the concentration of the solute in both phases no longer changes over time. - Chemical Affinity:
The absorption process relies on the chemical affinity between the solute and the absorbent. The solute molecules are more attracted to the absorbent than to the original phase, leading to their dissolution or capture by the absorbent material. The strength of this chemical affinity depends on the specific interactions between the solute and absorbent molecules, such as hydrogen bonding, ion-dipole interactions, or Van der Waals forces.
- Equilibrium and Saturation:
As mass transfer continues, the concentration of the solute in the absorbent phase gradually increases. Eventually, a point is reached where no further transfer of solute occurs, and the absorbent becomes saturated. At this stage, the absorbent phase contains the maximum amount of solute it can hold at the given conditions, and the system is considered at equilibrium.
- Contact between Phases:
- Desorption (Optional):
In some cases, it may be necessary to recover the solute from the saturated absorbent for further use or processing. This is achieved through a process called desorption, which involves changing the conditions (e.g., temperature, pressure, or chemical composition) to reverse the absorption process. Desorption releases the solute from the absorbent, making it available for collection or further treatment.
- Regeneration (Optional):
If the absorbent can be regenerated and reused, additional steps may be involved to separate the solute from the absorbent and return the absorbent to its original state. Regeneration processes can vary depending on the absorbent material and the nature of the solute.
- Product Recovery (Optional):
After desorption and regeneration, the recovered solute may undergo further processing steps to isolate and purify it. This can include distillation, crystallization, or other separation techniques depending on the specific application.
-
- Key Factors Influencing Absorption:
Several factors affect the efficiency and effectiveness of the absorption process:-
- Temperature:
Temperature plays a significant role in absorption. In general, higher temperatures can increase the solubility of gases in liquids but may also affect the overall equilibrium conditions.
- Pressure:
Pressure can influence the solubility of gases in liquids. Higher pressures can lead to increased gas solubility, which is a fundamental principle in processes like gas absorption and gas-liquid reactions. - Chemical Compatibility:
The choice of absorbent should consider its chemical compatibility with the solute and the overall process conditions.
- Contact Time:
The contact time between the phases affects the rate of mass transfer. Longer contact times may lead to higher solute absorption but may not always be practical.
- Absorbent Properties:
The physical and chemical properties of the absorbent, such as surface area, porosity, and selectivity, play a crucial role in the absorption process.
- Solvent Selection (for liquid-phase absorption):
In liquid-phase absorption, the choice of solvent can greatly impact the process’s efficiency and selectivity.
- Temperature:
The absorption process is widely used in various industries, including chemical manufacturing, environmental protection, gas purification, and pharmaceuticals. Its effectiveness depends on a careful selection of absorbents, process conditions, and optimization of various parameters to achieve the desired separation or purification goals.
-
- Advantages of Absorption:
Absorption processes offer several advantages for removing metal contents from kraft lignin:- Selective Removal:
Absorption allows for the highly selective removal of specific metal ions from the lignin solution. The choice of absorbent can be tailored to target particular metal contaminants.
- Efficiency:
Absorption is known for its high efficiency in metal removal, even at low concentrations. - Wide Applicability:
It can be applied to a variety of metal ions, making it versatile for different lignin solutions with varying metal content.
- Minimal Chemical Usage:
Absorption typically requires minimal or no chemical additives, reducing the environmental impact and operating costs. - Cost Considerations:
While ultrafiltration and membrane processes can be cost-effective in the long run, the initial capital investment for membrane systems can be significant.
- Scalability:
Absorption systems can be easily scaled up or down to accommodate different production volumes, making them suitable for industrial applications.
- Selective Removal:
- Challenges and Considerations
While absorption processes offer numerous advantages, they also come with specific challenges and considerations in the context of removing metal contents from kraft lignin:-
- Choice of Absorbent:
Selecting the appropriate absorbent is crucial for the success of the absorption process. Factors to consider include the absorbent’s selectivity, toxicity, environmental impact, and cost.
- Optimization:
Process parameters such as temperature, contact time, and absorbent-to-feed ratio need to be optimized to maximize metal removal efficiency. - Regeneration of Absorbent (Optional)
If metal recovery is required, the absorbent may need to be regenerated to remove the metal ions. This can involve using a different solvent or changing conditions.
- Safety:
Safety precautions should be taken when working with absorbents, especially those that may be toxic. Proper handling practices and disposal methods are essential.
- Choice of Absorbent:
-
- Recent Advancements and Research
Researchers and industry experts are continuously working on advancements in solvent extraction processes for metal removal from kraft lignin. Some recent developments and research areas include:-
- Green Solvents:
Exploration of environmentally friendly and biodegradable solvents to reduce the environmental impact of the extraction process.
- Selective Extractants:
Development of novel extractants with high selectivity for specific metal ions commonly found in kraft lignin. - Hybrid Processes:
Combining solvent extraction with other separation techniques, such as membrane filtration or adsorption, to enhance metal removal efficiency.
- Process Integration:
Integration of solvent extraction into existing kraft lignin production processes to streamline metal removal.
- Continuous Flow Systems:
Development of continuous flow solvent extraction systems for improved process control and efficiency.
- Innovative Regeneration Methods:
Research into innovative methods for regenerating organic solvents, reducing waste, and improving cost-effectiveness.
- Conclusion:
Solvent extraction processes are effective and versatile techniques for removing metal contents from kraft lignin solutions. They offer advantages such as selective removal, efficiency, wide applicability, and minimal chemical usage. However, the choice of solvent, optimization of process parameters, and environmental considerations must be carefully addressed to ensure successful metal removal while maintaining the integrity of the lignin product. Recent advancements in solvent selection, green extraction methods, and process integration are contributing to the ongoing development and improvement of solvent extraction processes for kraft lignin purification. As the demand for high-quality lignin continues to grow in various industrial applications, solvent extraction will play a crucial role in enhancing lignin purity and utility.
- Green Solvents:
-
Solvent Extraction Processes for Removing Metal Contents from Kraft Lignin
Solvent extraction is a separation technique commonly used to remove metal ions and other impurities from kraft lignin solutions. Kraft lignin, a byproduct of the pulp and paper industry, often contains metal contaminants that need to be eliminated to meet purity requirements for various applications. Solvent extraction processes offer an effective way to achieve this goal. In this comprehensive discussion, we will explore the principles, mechanisms, applications, advantages, challenges, and recent advancements in solvent extraction processes for metal removal from kraft lignin.
- Principles of Solvent Extraction
Solvent extraction is based on the principle of differential solubility, which relies on the varying solubilities of different substances in two immiscible phases—usually an organic solvent and water. The process involves several key steps:- Contacting Phases:
The kraft lignin solution (aqueous phase) containing metal ions is mixed or contacted with an organic solvent phase. The solvent is selected based on its ability to selectively dissolve the target metal ions.
- Distribution Equilibrium:
When the two phases come into contact, metal ions from the aqueous phase migrate into the organic phase, driven by their preferential affinity for the organic solvent. This migration continues until an equilibrium is reached. - Phase Separation:
After equilibrium is established, the two phases are allowed to separate, typically through settling or centrifugation. The metal-laden organic phase, known as the “loaded” organic phase, is separated from the aqueous phase.
- Phase Separation:
After equilibrium is established, the two phases are allowed to separate, typically through settling or centrifugation. The metal-laden organic phase, known as the “loaded” organic phase, is separated from the aqueous phase. - Stripping and Recovery:
The metal ions can be stripped or removed from the loaded organic phase by introducing a different aqueous solution. This new solution, known as the stripping or eluting solution, has a higher affinity for the metal ions than the organic solvent. The metal ions migrate back into the aqueous phase, leaving the organic phase “stripped” of metal impurities.
- Regeneration:
The stripped organic solvent can be regenerated and reused for further extraction cycles. Regeneration typically involves removing any residual aqueous phase and entrained impurities from the solvent.
- Contacting Phases:
- Applications of Solvent Extraction in Kraft Lignin Purification:
Solvent extraction processes have several important applications in the removal of metal contents from kraft lignin:-
- Lignin Recovery:
Solvent extraction can be used as a method to recover and purify lignin from black liquor, a byproduct of the kraft pulping process. Metal removal is an essential step in this process to ensure the quality of the recovered lignin.
- Purification:
Solvent extraction is employed to purify kraft lignin solutions obtained from various sources. It helps in achieving high lignin purity by selectively removing metal ions and other impurities - Purification:
Absorption is employed to purify kraft lignin solutions obtained from various sources. It helps in achieving high lignin purity by selectively removing metal ions and other impurities.
- Pre-treatment:
Solvent extraction can serve as a pre-treatment step before other downstream processes, such as membrane filtration or precipitation. By reducing the metal content in the lignin solution, these subsequent processes can be more efficient and cost-effective.
- Concentration:
In some cases, solvent extraction can be used to concentrate kraft lignin solutions. By removing water through the organic phase, the lignin content can be increased, making it suitable for specific applications.
- Lignin Recovery:
-
- Working Mechanism of Solvent Extraction
Solvent extraction is a separation process used to selectively remove specific components from a mixture based on their differential solubility in two immiscible phases—typically an organic solvent and an aqueous solution. This process relies on the principle that different substances will partition into different phases depending on their solubility, allowing for the separation of the desired components from the mixture. Here’s a detailed explanation of the working mechanism of solvent extraction:-
- Selection of Solvent and Aqueous Phase:
The first step in solvent extraction is selecting an appropriate solvent and an aqueous phase that are immiscible, meaning they do not mix together. The choice of solvent depends on the target compound you want to extract and its solubility properties. The aqueous phase typically contains the mixture from which you wish to extract the desired component.
- Mixing of Phases:
The aqueous phase, which contains the mixture with the target compound, and the organic solvent are mixed together. This mixing can occur through various methods, such as agitation or shaking. During this step, the two phases come into contact and create an interface between them. - Equilibrium and Partitioning:
As the two immiscible phases come into contact, the solutes (compounds) present in the aqueous phase begin to partition or distribute themselves between the two phases based on their solubility characteristics. The target compound, which is more soluble in the organic solvent, starts to migrate from the aqueous phase into the organic phase. The rate of partitioning depends on factors like the solubility of the compound, the volume of the solvent, and the temperature.
- Phase Separation:
Once the partitioning process reaches equilibrium, the two phases are allowed to settle or separate. The organic phase, now containing the target compound dissolved in the solvent, forms the “loaded” organic phase. - Stripping and Recovery:
The loaded organic phase is subjected to another phase separation, this time with a different aqueous solution known as the stripping or eluting solution. The stripping solution has a higher affinity for the target compound than the organic solvent. As a result, the target compound migrates from the organic phase into the stripping solution, leaving the organic phase “stripped” of the target compound.
- Regeneration of Organic Solvent:
The organic solvent, now free of the target compound, can often be regenerated and reused in subsequent extraction cycles. This regeneration typically involves removing any residual water or aqueous phase from the solvent.
- Recovery of Target Compound:
The target compound is now present in the stripping solution. Depending on the specific application, it may undergo further processing to recover the compound in its pure form. This can include methods like evaporation, crystallization, or additional separation techniques.
- Recycle or Disposal:
The aqueous phase left after stripping the target compound from the organic phase may contain other components from the original mixture. Depending on the composition and environmental regulations, this aqueous phase may be recycled, treated, or disposed of in an appropriate manner.
- Selection of Solvent and Aqueous Phase:
-
- Key Factors in Solvent Extraction:
Several factors influence the effectiveness of solvent extraction:-
- Solvent Choice:
The selection of the right solvent is critical. It should have a high affinity for the target compound and should be immiscible with the aqueous phase. The solvent should also be compatible with the target compound and other components in the mixture.
- Temperature:
Temperature can significantly impact the solubility of compounds in both the solvent and the aqueous phase. Controlling the temperature can enhance the efficiency of the extraction process. - pH:
The pH of the aqueous phase can influence the solubility of compounds. Adjusting the pH may be necessary to optimize extraction efficiency.
- Extraction Time:
The duration of the mixing and equilibration stages can affect the extent of extraction. Longer contact times may lead to greater partitioning of the target compound.
- Solvent-to-Feed Ratio:
The ratio of solvent to the volume of the aqueous phase (often referred to as the solvent-to-feed ratio) can influence extraction efficiency. A higher ratio can sometimes improve extraction, but it also affects the volume and cost of solvent required.
- Safety:
Safety precautions should be taken when working with organic solvents, especially those that may be toxic or flammable. Proper ventilation, protective equipment, and safe handling practices are essential
- Solvent Choice:
Solvent extraction is a versatile and widely used separation technique in various industries, including chemical processing, pharmaceuticals, mining, and metallurgy. Its success depends on careful consideration of the solubility properties of the compounds involved and the selection of appropriate solvents and process conditions. Solvent extraction offers a valuable tool for purifying and separating compounds, including the removal of metal contents from solutions like kraft lignin in the pulp and paper industry.
-
- Advantages of Solvent Extraction:
Solvent extraction processes offer several advantages for removing metal contents from kraft lignin:- Selective Removal:
Solvent extraction allows for highly selective removal of specific metal ions from the lignin solution. The choice of solvent can be tailored to target particular metal contaminants.
- Efficiency:
Solvent extraction is known for its high efficiency in metal removal, even at low concentrations. - Wide Applicability:
It can be applied to a variety of metal ions, making it versatile for different lignin solutions with varying metal content.
- Wide Applicability:
It can be applied to a variety of metal ions, making it versatile for different lignin solutions with varying metal content. - Minimal Chemical Usage:
Solvent extraction typically requires minimal or no chemical additives, reducing the environmental impact and operating costs.
- Scalability:
Solvent extraction systems can be easily scaled up or down to accommodate different production volumes, making them suitable for industrial applications.
- Regeneration:Organic solvents used in the process can often be regenerated and reused, enhancing cost-effectiveness
- Selective Removal:
- Challenges and Considerations
While solvent extraction processes offer numerous advantages, they also come with specific challenges and considerations in the context of removing metal contents from kraft lignin:- Choice of Solvent:
Selecting the appropriate solvent is crucial for the success of the extraction process. Factors to consider include the solvent’s selectivity, toxicity, environmental impact, and cost.
- Solvent Compatibility:
Solvents must be compatible with the lignin and other components in the solution to prevent undesirable interactions or changes in lignin properties. - Optimization
Process parameters such as temperature, contact time, and solvent-to-feed ratio need to be optimized to maximize metal removal efficiency.
- Regeneration and Waste Management:
The regeneration of organic solvents and proper disposal or treatment of waste streams generated during the process are important considerations for environmental sustainability.
- Residual Solvent:
Ensuring that the final lignin product is free from residual solvent is critical, especially if the lignin will be used in applications where solvent traces are undesirable. - Scale-Up Challenges:
Scaling up solvent extraction processes for industrial applications may involve complex engineering and logistical challenges
- Choice of Solvent:
- Recent Advancements and Research
Researchers and industry experts are continually working on advancements in absorption processes for metal removal from kraft lignin. Some recent developments and research areas include:-
- Green Absorbents:
Exploration of environmentally friendly and biodegradable absorbents to reduce the environmental impact of the absorption process
- Selective Absorbents:
Development of novel absorbents with high selectivity for specific metal ions commonly found in kraft lignin. - Hybrid Processes:
Combining absorption with other separation techniques, such as membrane filtration or adsorption, to enhance metal removal efficiency.
- Process Integration:
Integration of absorption into existing kraft lignin production processes to streamline metal removal.
- Continuous Flow Systems:
Development of continuous flow absorption systems for improved process control and efficiency.
- Innovative Regeneration Methods (Optional):
Research into innovative methods for regenerating absorbents, reducing waste, and improving cost-effectiveness, particularly when metal recovery is needed.
- Green Absorbents:
Absorption processes are effective and versatile techniques for removing metal contents from kraft lignin solutions. They offer advantages such as selective removal, efficiency, wide applicability, and minimal chemical usage. However, the choice of absorbent, optimization of process parameters, and environmental considerations must be carefully addressed to ensure successful metal removal while maintaining the integrity of the lignin product. Recent advancements in absorbent selection, green absorption methods, and process integration are contributing to the ongoing development and improvement of absorption processes for kraft lignin purification. As the demand for high-quality lignin continues to grow in various industrial applications, absorption will play a crucial role in enhancing lignin purity and utility.
-
Waterman Engineers Australia’s Offering of All These Processes
Waterman Engineers Australia, as a company offering various processes for metal removal from kraft lignin and potentially other applications, may indeed have advantages over other options available on the market. These advantages could stem from factors such as innovation, technology, expertise, or unique solutions tailored to specific customer needs. Here are some potential advantages that Waterman Engineers Australia might offer:
-
- Customized Solutions:
Waterman Engineers Australia may provide tailored solutions to meet the unique needs of their clients. They could assess the specific requirements, challenges, and goals of a particular project and design processes accordingly.
- Customized Solutions:
-
- Expertise and Experience::
The company may have a team of experienced engineers, scientists, and experts who are well-versed in the field of metal removal and separation processes. Their knowledge and experience can be invaluable in developing efficient and effective solutions.. - Innovative Technologies:
Waterman Engineers Australia might invest in research and development to stay at the forefront of technology. This could lead to the development of new and advanced processes for metal removal, potentially offering superior performance compared to traditional methods.
- Efficiency and Cost-Effectiveness:
Their processes may be designed for high efficiency, reducing the consumption of resources such as energy and chemicals. This efficiency could translate into cost savings for their clients.
- Environmental Considerations:
Waterman Engineers Australia may prioritize environmentally friendly processes that produce minimal waste and emissions, aligning with sustainability goals and regulatory requirements.
- Innovative Regeneration Methods (Optional):
Research into innovative methods for regenerating absorbents, reducing waste, and improving cost-effectiveness, particularly when metal recovery is needed.
- Scalability:
Their solutions may be designed to be scalable, making them suitable for both small-scale and large-scale industrial applications. - Regulatory Compliance:
Waterman Engineers Australia might ensure that their processes and technologies comply with relevant industry standards and regulations, reducing compliance-related risks for their clients.
- Technical Support and Maintenance:
They could offer ongoing technical support and maintenance services to ensure that their clients’ processes continue to operate efficiently.
- Expertise and Experience::
-
- Global Reach:
If they have a global presence or partnerships, Waterman Engineers Australia might offer their solutions to clients in various regions, providing access to their expertise and technologies worldwide.
- Customer-Centric Approach:
Their customer-centric approach may prioritize strong communication, collaboration, and a focus on delivering value to clients throughout the project lifecycle.
- Global Reach:
It’s important to note that the specific advantages offered by Waterman Engineers Australia would depend on their unique capabilities, innovations, and the details of their services and technologies. Clients interested in their offerings should engage with the company directly to explore how their processes can address specific metal removal needs and deliver the desired benefits. Additionally, conducting a comparative analysis of Waterman Engineers Australia’s solutions against other available options in the market can help clients make informed decisions based on their specific requirements and objectives