Nanotechnology- Adsorption kinetics to treat industrial wastewater
Introduction:
Due to excessive use of water in the manufacturing of industrial products, it puts a lot of pressure on the water reserves. Therefore, it is important to treat the waste water to reuse it or dispose it. Various techniques re used to treat the toxicity of effluents in the waste water like electrochemical process, advanced oxidation process and valorisation, but these Effluent treatment plants techniques are not cost effective. With the development of nano-technology and nanoscience new advances are made in the treatment of wastewater that are cost effective and efficient. Because nanoparticles are smaller in size, have high surface area to volume ratio and superior chemical properties, they are more suitable for treatment of wastewater. Moreover, because of their other advantages like superparamagnetic, semiconducting, and quantum confinement effect, they are very effective. Harmful contaminants from industrial water can be removed by using nanoparticles as adsorbents. Organic and inorganic contaminants can both be removed using nano-adsorbents in wastewater recovery. These adsorbents are characterized as carbon based, metal and metal-oxide based. Carbon nanotubes (CNTs), activated carbon, graphene, and fullerene fall in the category of carbon-based nano-particles. Toxic chemicals that pollute water in manufacturing and pharmaceutical industries are removed by using carbon nanotubes as adsorbents. Fluoride ions from water are removed by using activated carbon modifies nano-magnets.
Metal and metal-bases nano-particles also play an important role in the treatment of wastewater. Adsorption efficiency of magnetic nano-particles can be increased by coating them with other supports. Moreover nano-particles are cost effective and environmentally friendly because they can be reused and are stable. When integrated with microorganisms and enzymes nano-particles provide a greener approach to the treatment of industrial wastewater.
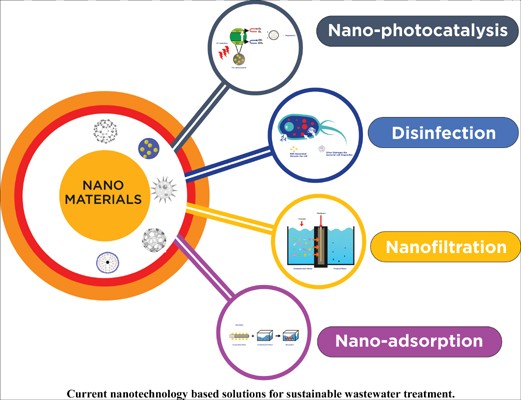
Wastewater treatment by nano-particles are divided into three categories based on the type of nanomaterial:
- Nano catalysts
- Nano adsorbents
- Nanomembranes
Nano catalysts: In this treatment photocatalytic activities are used in which light energy interact with metallic nanoparticles. Microorganisms and organic compounds in waste water destroyed by photocatalytic activities by reaction with hydroxyl radicals. Inorganic materials like metal oxides and semiconductors are used in nano catalysts.
Nano adsorbents: In this treatment process organic and inorganic nanomaterials are used that have high affinity to adsorb substances. Many contaminants can be removed efficiently using these adsorbents. Large surface area, high catalytic potential, and high reactivity are all characteristics of an excellent adsorbent. Metallic nanoparticles, magnetic nanoparticles, nanostructured mixed oxides, and metallic oxide nanoparticles are the four types of nano adsorbents.
Nano membranes: In this treatment nanomembranes are used to separate contaminants from water. Heavy metals, dyes, and other pollutants are commonly removed with these. Commonly used nano membranes are nanotubes, nanoribbons, and nanofibers.
Nano Adsorbents:
Overview:
Removal of organic and inorganic matter from through adsorption process utilizing carbon-based adsorbents is considered most effective. Adsorption is a surface phenomenon where organic and inorganic matter through adhesion attach to the surface of an adsorbent. Adhesion arises from physical-chemical forces caused dur to Van der Waals and electrostatic interactions.
An effective adsorbent need to be inert, resistant to mechanical forces, biocompatible and must have high adsorption capacity to ensure effective removal of waste from water. The adsorption process is influenced by factors such as pH, temperature, pollutant concentrations, contact time particle size, contact time, and the chemical and physical properties of the adsorbate and adsorbent. pH alters the surface groups present on the adsorbent and pollutant charge, influencing adsorbent capacity. Temperature increase can increase adsorption capacity in endothermic reactions. By increasing the contact duration, the amount of adsorbent is increased.
Carbon-based nano adsorbents:
These are cost effective, abundant, have high chemical and thermal stabilities, high active surface areas, excellent adsorption capacities, and environmentally friendly nature. Due to these properties these are most commonly used as adsorbents to treat wastewater. Because of its enormous surface area and high porosity, activated carbon is the most commonly utilized carbon-based nano adsorbent.
Polymer-based nano adsorbents:
These adsorbents are cost effective, allow fast decontamination due to large surface area, are remarkably stable and have improved processibility. Polymer-based adsorbents that are most commonly used in treatment of waste water are polysaccharides, namely CS cyclodextrin, nano-magnetic polymers, covalent organic polymers, extracellular polymeric substances etc. polymer derived from cellulose known as nanocellulosics are nontoxic, ubiquitous, excellent adsorbents. They’re employed in wastewater treatment since they’re easy to modify on the surface. Lignin derived nano materials are used in the catalytic degradation of dyes, nitroarenes, and heavy metal removal.
Metal based adsorbents:
Heavy metals, ions and dyes are removed from water by use of nanometals and their oxides. Commonly Fe3O4, TiO2, MnO2, MgO, ZnO and CdO are used for treatment of waste water. due to their small size and large surface area, nanometal oxides are more effective adsorbents than activated carbon and can effectively remove heavy metals and radioactive metals.
Zeolites:
These three-dimensional, crystalline microporous materials are used as adsorbents to treat waste water because they are stable in water, are cost effective, have high surface area and are environmentally friendly.
Clay based adsorbents:
Clay and clay-based adsorbents are used as clarifying agents for organic pollutants. Because they are richly composed of silica, alumina, iron, calcium, and magnesium oxides, they are also very effective in removing heavy metals from waste water.
Commonly used adsorbents: Most commonly available commercial adsorbents are:
- Activated alumina
- Silica gel
- Activated carbon
- Molecular sieve carbon
- Molecular sieve zeolite
- Polymeric adsorbents
Working:
Mechanical filtration is used to treat water. But extremely small particles cannot be effectively removed through mechanical filtration therefore adsorbents are used to remove these small contaminants.
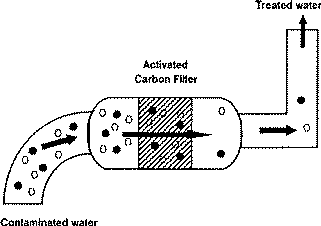
In adsorption process waste water is passed over an adsorbent which attracts and removes dissolved molecules and small particles from water. These impurities bind to the adsorbent, and the water is cleansed as a result. Attractive forces called van der Waals forces come into play during this process and cause the molecules of adsorbate to become attached to the adsorbent.
Activated carbon is the most often used adsorbent. It is an inert solid adsorbent capable of removing diverse dissolved contaminants from water. it can remove toxicity, odor, taste and color. The surface interaction between the contaminants and carbon graphitic surface removed the contaminants from water. Van der Waals forces and induced dipole interactions are involved in this interaction. Neutral organic molecules are induced into intra-molecular dipole by the activated carbon. The molecules are attracted to each other and stick together due to induced dipoles. This causes them to precipitate out of the water and into carbons nano sized pores.
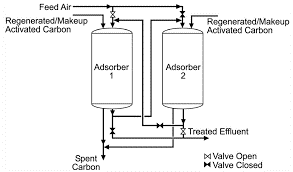
Adsorptive filters: Water is filtered using two types of filters particulate filters and adsorptive/reactive filter. Particulate filters work by removing particles from water depending on their size whereas adsorptive filters have a medium either adsorbs or reacts with the contaminants present and water and removed them. Most common adsorptive used in these filters is activated carbon. Activated carbon can either be point of use or point of entry treatment. For radon and volatile organic compounds point of use device is used because these contaminants can vaporize from water. A point of use device can be in-line, placed on a line-bypass faucet, or poured through. In water treatment plants, activated carbon is also employed as a tertiary treatment to remove micropollutants.
The adsorbent’s ability to remove pollutants is determined by factors such as particle and pore size, surface area, surface chemistry, and so on. Moreover, contaminant characteristic also influences adsorption efficiency. Contaminants that are less water soluble can be easily eliminated. Adsorption also depend on the affinity the contaminant has with the adsorbent.
| Working principle | The pollutants are removed from water through adsorption on the surface of the activated carbon. Use in a POE or POU situation (e.g. advanced filters). |
| Capacity/adequacy | It is a simple technique that uses abundant raw material (Petroleum coke, bituminous coal, lignite, wood products, coconut shells, and peanut shells, to name a few). At least periodically, skilled labor is required. |
| Performance | It is efficient for pollutant that has high affinity with activated carbon surface (non-polar compounds). |
| Costs | Relatively low operation costs. |
| Self-help compatibility | Initial analysis of water is required to choose proper adsorbent (type of activated carbon). |
| O&M | Carbon cartridges should be replaced or regenerated on a regular basis. |
| Reliability | Reliability When choosing the type of activated carbon to utilize as a filter material, the water composition should be considered. |
| Main strength | Activated carbon can be made reasonably cheaply almost anywhere in the planet. |
| Main weakness | Filter The filter must be changed on a regular basis. |
Table 1: Specifications of activated carbon adsorbent filter
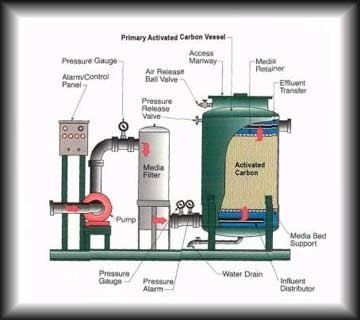
Arsenic treatment from ground water:
Preferred adsorptive material for removal of arsenic are compounds of iron like hematite, goethite, iron oxide-coated materials and granular ferric hydroxide. These have magnetic properties that allow them to efficiently remove arsenic from water and also lead to less leaching of arsenic from exhausted adsorbent. Cupric oxide also proved to be an efficient adsorbent for the removal of arsenic as it doesn’t require pH adjustments. Cupric oxide can be easily regenerated and reused. Titanium dioxide can also be used as an adsorbent as it is chemically stable, nontoxic, cost effective, have high affinity with arsenic and is corrosion resistant. Another adsorbent used is Zinc Oxide. It is used because it is non-toxic and stable. Some other adsorbents used include mixed metal oxides and carbon nano tubes.
Adsorption process:
The process of adsorption can either occur in a single step or in a combination of steps like film or external diffusion, pore diffusion, surface diffusion and adsorption on the pore surface. The adsorption process of arsenate proceeds in three steps
- Migration to adsorbent surface.
- Dissociation of complex aqueous arsenate or arsenite.
- Surface complexation.
During this process arsenate or arsenite diffuses on the external surface of nano adsorbent. This occurs due to diffusion potential that is characterized by concentrations of adsorbate and external surface availability of adsorbent. After diffusion of adsorbate on the adsorbent surface, it diffuses on the available pores of the adsorbent. During the adsorption process all the exposed sites are occupied.
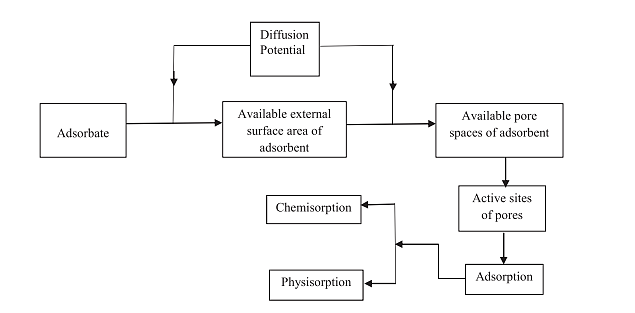
Application of nano adsorbent:
- One of the most effective water treatment methods
- Adsorbents that are effective at removing metals and colors from wastewater.
- Iron based nano adsorbents can remove toxic materials from aqueous solutions.
- Manganese oxide nano adsorbents are efficient for removing heavy metals.
- Inorganic pollutants can be easily removed from waste water by using zinc oxide adsorbent.
- Activated carbon adsorbent is used in carbon nano tubes for efficient removal of contaminants from waste water.
| S No. | Pollutants | Adsorbents (Nano particles | Removing capacities | Contact time | pH |
| 1 | As (III) | Cobalt (10 -20 nm) and manganese (10 – 50 nm) ferrite | 24.81 and 24.17 mg
g – 1 |
4 hr | 2 |
| 2 | As (III) and As (V) | Hematite | 2899 ± 71.09 µg g – 1 and 4122 ± 62.79 µg
g – 1 |
8 hr | 6-8 |
| 3 | As (V) | Iron NPs modified micro fibrillated cellulose | 2.460 mmol g-1 | 5 -600 min (75 min) | 2 |
| 4 | Cr (VI) | Graphene oxide -Cobalt oxide | 208.8 mg g-1 | 12 hr | 3 |
| 5 | Cr (VI) | Zero valent iron nanoparticles | 100% | 10-30 min | 2 |
| 6 | Cr (VI) | Copper Oxide | 15.62 mg g – 1 | 180 min | 3 |
| 7 | Cd (II) | Nanoscale zerovalent iron particles supported on reduced graphene oxide | 425.72 mg g – 1 | 50 min | 5 |
| 8 | Cd (II) | Ascorbic acid -stabilized zero valent iron nanoparticles | 79.58% | 60 min | 7 |
| 9 | Cd (II) | γ -Al2O3 NPs | 17.22 mg g – 1 | 30 min | 5 |
| 10 | Pb (II) | Magnetite Fe3O4/ Chitosan nanoparticles (Fe3O4 / CSNPs | 79.29 mg g -1 | 12 hr | 6 |
| 11 | Pb (II) | Sulfonated magnetic NPs | 108.93 mg g – 1 | 24 hr | 7 |
| 12 | Pb (II) | MgO | 2614 mg m – 1 | 180 min | – |
| 13 | Cu (II) | Modified henna with Fe3O4 | 99.11% | 85 min | 4 |
| 14 | Cu (II) | Nanocomposite of ZnO with montmorillonite | – | 90 min | 4 |
| 15 | Cu (II) | γ -alumina | 31.3 mg g – 1 | 4 hr | 5 |
| 16 | Ni | γ -alumina NPs and MWCNTs | 99.41% and 87.65% | 30 min | 10 |
| 17 | Ni | Superparamagnetic Fe3O4 | 209.205 to 362.318 mg g – 1 | 35 min | 8 |
| 18 | Ni | Aluminium substituted goethite (Al – FeOOH) | 94.52 mg g – 1 | 6 hr | 5 |
| 19 | Th (I) and Th (III | Titanate nanotubes | 709.2 mg g – 1 | 10 min | – |
| 20 | Pb (II), Cd (II), Cu (II), Ni (II) | Nano scale zero valent iron particles (nZVI) | – | 5 -600 min (75 min) | 2-7 |
Table 2: Removal of inorganic water pollutants by adsorption using Nano -adsorbents
Advantages:
- They have short reaction time and rapid separation of pollutants.
- Can be regenerated and reused without altering their removal performance.
- Pollutant removal from wastewater is more cost-effective and efficient.
- Capability to minimize generation of secondary waste using less resources.