Revitalizing Resources: Promoting Caustic Soda (NaOH) Recovery in the Textile Industry
Caustic is a strongly alkaline and hygroscopic substance. Caustic soda is a white, solid ionic chemical composed of sodium (Na+) and hydroxide ions (OH–). It is also known as lye. When dissolved in water, it generates a solution with a high pH. Chemically, caustic solutions have the ability to dissolve organic materials hydrocarbons. It is widely employed on the market for cleaning process equipment and other purposes.

Caustic is used in many industries in many applications. The biggest users of caustic are the pulp, paper, and textile industry. The Pulp, paper industry caustic is used to purify fibres, cellulosic fibres by removing dissolving all kinds of organic contaminants. In the textile industry, similar application of removing contaminations from rayon fibres before it goes to the fabric manufacturing and mercerization.
The mercerization process is charged with caustic is used for treatment of cotton fabric to improve the lustre of the fabric and to improve the affinity to dyes and these are in all of these applications very high concentrated of caustic is used. Uses of Caustic are in the food dairy and beverage industry where caustic is used to clean equipment to make sure that the process equipment is clean for the next batch. In pharmaceutical industry, caustic is used for element to high value components materials and for regeneration of residence in various applications.
In most cases caustic is simply going to drain to the wastewater treatment plant to be treated as part of the overall wastewater of the plant. Caustic is an expensive chemical it’s the price varies depending on manufacturing and production, but it’s overall between 300 to 900$ per tonne of solid 100% caustic. So, recovering of caustic before it goes to the drain and before it goes to the wastewater treatment plant can save a lot of money to the user. It also saves water energy and overall, the cost saving for the recovery of caustic is to the manufacturer is very high
It is a caustic when it goes to the wastewater treatment plant it increases the pH of the wastewater often this makes difficult wastewater treatment process. So, before caustic is sent to the wastewater treatment plant it needs to be neutralised and by recovering the caustic before it goes to the wastewater not only the caustic is being saved, but also the acid consumption for neutralisation is being reduced. In addition, the overall carbon footprint of the plant is reduced and there are savings by reducing the flows to the wastewater treatment.
It’s interesting because the effluent that recovered caustic from the membranes is better than the raw caustic that buy originally. Overall, the reduction from the caustic of organic matter is almost complete the caustic is clear, and the caustic is at the same concentration of what was fed into the membered system.
There is a complete solution, also a family of systems in market for recovering the caustic from mercerization. Available membrane is based on the central product, this is a nanofiltration membrane that not only has an optimal separation property to pass the cost they can retain all the contamination, but it also has the stability that is required to withstand these high pH conditions and often also high temperature conditions. This membrane is a spiral bound element around it. Designed system family the caustic of systems that offer solutions for small medium and large flow rates for recovery of caustic.
These are very harsh conditions. Typical caustic is used in the industry, anything between 1-20% and the temperatures can go as high as 70 degrees centigrade or 160 Fahrenheit. The combination of high concentrated caustic at high temperature or very corrosive harsh most materials fail in short time. The construction materials of the systems and membranes have to be very carefully picked in order to serve and provide long life of product in the industry. Typical life can be between 1-5 years depending on the cost the concentration and depending on what is to be removed from the cost state, but a good number is 1 to 5 years.
Smallest system typically treats around 5000 gallons per day or one cubic metre per hour. It is based on one single pressure vessel with loaded with spiral bound elements. Their largest system is designed to treat 250,000 gallons per day. For larger flow rates, we can either customise the larger system or provide multiple scales of the large systems.
These automated systems operating mode varies from the small systems to the large systems, the nature of the small systems is more, they are more manual, that’s basically what the client wants from a very small system, they operate in a batch mode, meaning that we have a tank it contains caustic, contaminated caustic, we run a batch mode, we treat this caustic and then clean the membrane and move on to the next day. The medium sized systems have a lot more automation they are kind of what we defined semi-automatic, they can run at both batch mode or continuous mode and the large systems are fully automated with full controls and they run in a fully continuous operating mode.
Principal of Working
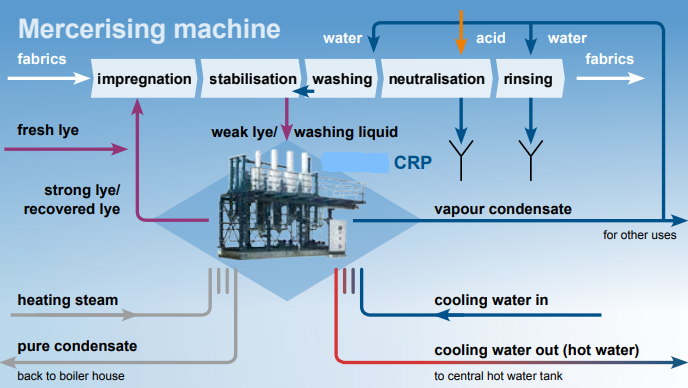
Using a heat source, the caustic is heated to its boiling point, and the vapours formed by boiling are utilised to heat the weak liquid. The resulting vapours are then reused. The diluted liquid is concentrated to 250-300 gpl in dry Mercerized and 450-500 gpl in wet Mercerized, at which point it can be reused with moderate caustic loading.
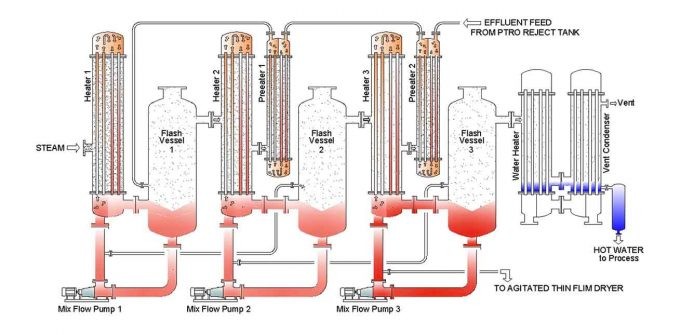
Explanation of working
From the impregnation and washing chamber, 5 to 6 Be (40 to 50 gpl) of weak caustic lye is collected in a storage tank. In a filtration unit, the suspended particles, fluff, and other waste are subsequently filtered. The feed to CRP then passes through a series of preheaters that utilize flash vapour from condensate flashes to preheat the wash liquor, resulting in little steam loss while heating the wash liquor to its boiling point. The liquid in the tubes contacts the steam on the shell side of heater 1 and begins to boil. The vapor-liquor mixture enters the flash vessel tangentially, where the vapour separates and enter the second-stage shell.
With the assistance of a water ring vacuum pump, a decrease in pressure generates the boiling effect. Due to the decreased pressure, the boiling point of the liquid is lowered, and it begins to boil. Due to the pressure difference, the concentrated liquid enters the second heater and comes into indirect contact with the created vapour; the process is then repeated. A product pump removes the final step’s concentrated product. This is then either stored or refined using a procedure involving concentrated lye.
 The final stage of the caustic recovery system / caustic soda recovery plant must condense the generated vapour. To do this, either hot water or an adiabatic evaporator system is utilized. In a hot water system, water at room temperature is transferred through tubes of an exchanger, where it is heated by vapour. Since this hot water is untainted, it can be utilized in the process or boiler without risk.
The final stage of the caustic recovery system / caustic soda recovery plant must condense the generated vapour. To do this, either hot water or an adiabatic evaporator system is utilized. In a hot water system, water at room temperature is transferred through tubes of an exchanger, where it is heated by vapour. Since this hot water is untainted, it can be utilized in the process or boiler without risk.
The effluent is conveyed through the exchanger and sprayed in an adiabatic evaporator when the produced hot water cannot be utilized appropriately. Waste heat is lost as vapour, resulting in a decrease in effective steam utilization. Also eliminated is the nuisance of very hot water.
Applications
Caustic concentration has use in all industries where washing generates dilute caustic, and the concentrated liquid can be reused under the caustic recovery system/caustic soda recovery plant. Knit mercerizing, for instance, has relatively few impurities such as size and starch, therefore the caustic that is recovered is equally clean. Before contaminants accumulate to undesirable levels, reclaimed caustic can be utilized for a longer period of time.
The weak liquor in grey knit mercerizing is relatively large, thus the recycled caustic must be replaced approximately every 15 days. In addition, the origin and quantity of the sizing substance are largely unknown. Before the recovered caustic can be utilized, it must be treated due to its viscosity and low gpl. This recovered caustic must be dosed with new caustic in order to retain its gpl.
Who can Install This Caustic Recovery System?
If you are user and that you purchase caustic in large quantities and you dispose caustic in large quantities to your wastewater treatment and most likely also use acid to neutralise this caustic and you use the caustic at 1% concentration or higher, most likely you’re a very good candidate for caustic recovery system. Overall, you will save money on purchasing less caustic and let’s not forget the environmental impact of recovering caustic. Caustic has a lot of sodium in it and disposing sodium into the environment is not a good thing. It’s a global goal to reduce disposal and use of sodium. So, recovering caustic is basically improvement of environmental sustainability and if we can help the environment and save money, this is a win situation.
It is Worth to Recover Caustic Soda
To prepare, clean, and colour materials, the textile industry makes extensive use of chemicals. Caustic soda (NaOH) is one of the most often utilised compounds to improve the strength and shine of cellulosic materials. Cotton is dipped into a concentrated caustic soda solution and additional wetting agents under tension at room temperature during the mercerizing process. The mercerized fabric is rinsed with water at the end of the mercerizing cycle to remove any surplus caustic. The rinse water, which is mostly weak caustic, is typically produced in large quantities, and if discharged without treatment, can result in a significant loss of caustic soda and a high consumption of acid for neutralisation in the Effluent Treatment Plant (ETP), resulting in large volumes of sludge.

It is feasible to recover used caustic from the effluent stream by installing a Caustic Recovery Plant (CRP). CRPs use heat exchangers to concentrate weak caustic in effluent streams, evaporating superfluous water from the solution and leaving concentrated caustic behind. CRPs might have numerous steps to enhance the concentration of the recovered caustic soda to the required amount.
The created vapour is condensed with non-contact cooling water at the end of the recovery process, yielding hot water that can be used in other process units such as bleaching units and boiler feed tanks. The number of CRP stages must be adjusted to accommodate the necessary amount of hot water since textile mills sometimes demand enormous amounts of hot water. A three-stage evaporation plant may occasionally be more economical than a four-stage unit.
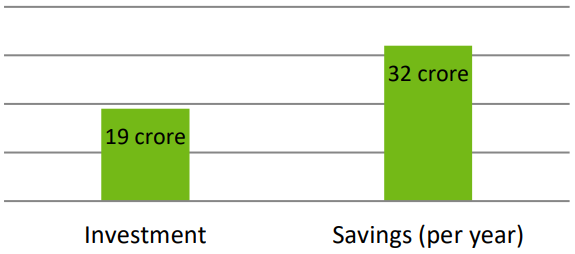
According to data from the Bangladesh textile industry, a four-stage CRP with a capacity of 38,000 litres per day can recover 6.5 million litres of caustic soda per year at a concentration of 28 °Baumé. A by-product of the recovery process is approximately 28 million litres of hot water each year. Annual savings from reduced caustic soda purchases and reduced hot water usage are projected to be around BDT 32 crore. CRPs typically have a payback period of less than one year because the total investment costs are anticipated to be below 19 crores.
Advantages of Chemical Recovery
- Reduced waste caustic discharge and effluent treatment costs
- Savings from reduced purchases of caustic soda
- Saving in energy cost for heating water
Why is a Caustic Recovery Plant Essential?
After food, clothing is the second most essential human need. The fundamental milestones of the textile industry are the production of fibres and their subsequent transformation into clothes. Because of this unavoidable need, the textile industry has developed at a quicker rate as the population and living standards have risen over time. As a result, the textile industry has established itself near countries’ biggest export and import markets.
Typically, textile processing begins with yarn formation, then moves on to yarn production, and eventually dyeing and finishing. The most essential production phases of the textile business are dyeing and finishing, sometimes known as “wet processing.” Wet processes enhance the value of fabric in terms of aesthetics, durability, and functionality, taking into account the needs of the client. However, owing of these processes, the textile industry is extremely water- and chemical-intensive. In a typical textile industry, between 200 and 400 litres of water are utilised to produce 1 kilograms of fabric, resulting in substantial quantities of effluent.
Textile wastewaters are brightly coloured and include significant levels of inorganic and organic pollutants. Moreover, due to the textile industry’s reliance on product base trends, the quality and quantity of these wastewaters are highly variable. As a result, chemicals or additives utilised in processes undergo significant changes over time. In textile effluents, almost 8000 colourants and 6900 addition pollutants contribute to organic and inorganic contamination.
Textile firms frequently encounter water shortages due to both water scarcity and discharge restrictions. These facts have diverted the textile industry’s attention to water conservation and recovery methods, meaning that end-of-pipe treatment of textile wastewaters is useless.
Membrane approaches are generally recognised as feasible choices for the recovery-oriented treatment of textile wastewaters. Traditional approaches (like biological degradation of activated sludge) fall short of not only achieving the necessary water quality but also in terms of water and chemical recovery.
The main chemical used in the mercerization procedure, which is an essential finishing step in the manufacture of cotton textiles, is caustic (NaOH). This method is widely used to improve the fibber’s strength, gloss, and dye affinity. To get rid of extra caustic wherever it is used, the mercerization procedure is followed by an extensive hot water rinse or washing operation. The result is the formation of an effluent that is both hot and extremely alkaline (60–70 g/L NaOH), as well as containing a variety of other organic and inorganic contaminants.
Wastes from Mercerizing Processes
Textile mills face challenges with very alkaline wastewater and substantial caustic soda losses due to waste from mercerizing processes. However, there is a possibility of recovering caustic from these wastewaters. In actuality, there are two distinct caustic recovery methods for mercerizing wastewater. Since 1900, the staged evaporation process has been utilised in the textile and nonwoven industries. Using this approach, the caustic in the wastewater is totally recovered. Despite the fact that there are good examples of this system, it is unsuitable for some producers due to operational challenges brought on by the wide variety of chemicals employed.
Membrane-based Caustic Recovery Methods
In boiling water, surfactants, which are used in virtually all textile processes and by-products, can produce foaming concerns. Consequently, membrane-based caustic recovery methods from mercerizing wastewaters have been devised. Membranes have proved their utility in caustic recovery and a variety of textile wastewater treatment applications. However, caustic recovery by membrane techniques does not ensure 100% caustic or NaOH recovery from alkaline wastewaters. The input to the membrane recovery system generates a permeate containing caustic that is subjected to a subsequent evaporation stage.
UF is recognised by the membrane technology as a caustic recovery technique. Although caustic has been recovered from mercerizing process effluents using this method, it is generally considered sufficient for particle and macromolecule removal; nevertheless, additional treatment is normally required for decolorization. However, NF may be used to decolorize and separate organic molecules with low molecular weights as well as divalent ions. Additionally, it has a unique softening effect.
Therefore, the use of NF for caustic recovery is preferable. In a few recent research, NF was utilised efficiently to recover caustic from cotton and polyester fabric manufacturing mercerizing process effluents.
Although effective caustic recovery was accomplished, membrane performance was not evaluated using pollutant metrics such as COD or colour removal. Furthermore, in both experiments, only one type of NF membrane was tested. Due to the nature of mercerizing processes, high-temperature scenarios should also be evaluated for treatment options. However, none of these studies investigate the effect of temperature on membrane performance.

Fabric Preparation
Fabric pre-treatment is utilised to enhance wetting capacity and absorption, dye uptake capability, fabric purity, lightening, and material development. The position of the pre-treatment can vary depending on the dying scheme. In addition, the pre-treatment techniques utilised depend on the fabric’s characteristics, such as the type of fibre, the form of fibre, and the quantity of fibre to be treated.
There are primarily five pre-treatment methods.
(i)Mercerizing, (ii) Scouring, (iii) De-sizing, (iv) singeing, and (v) Bleaching.
Singeing is a dry procedure for removing projecting fibre ends from yarns or fabrics. The filaments are eliminated by passing them through a flame or copper plates heated to high temperatures. Singeing is a necessary process if a smooth end product is required. Another preparation stage is de-sizing, which removes previously applied sizing ingredients. Prior to weaving and knitting, sizing should be applied. However, de-sizing is required to avoid sizing materials reacting with chemicals that will be used in subsequent procedures.

Scouring is a cleaning procedure that is used to remove contaminants from fibres or yarns. This technique mostly uses alkaline solutions (sodium hydroxide), but it can also employ solvent solutions. Bleaching is also a chemical pre-treatment technique for removing substances from fibres or yarns that affect whiteness. Several bleaching chemicals, including hydrogen peroxide and sodium hypochlorite, are utilised to decolorize fibres or yarns in preparation for dying or printing.

Mercerization Process
Mercerization is an alkaline treatment that is used to give cotton a permanent shine and improve handling in both the pre-treatment and finishing stages of manufacture. The following reaction Shows the application of mercerizing and its effects on cloth at various stages of manufacture.
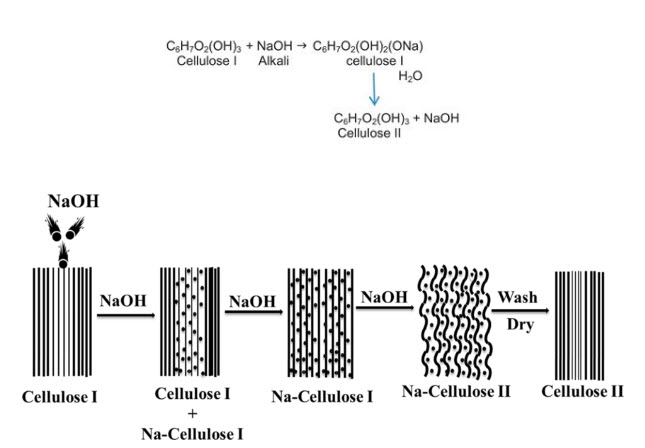
According to mercerization, “the look of the fabric is improved by making it shinier, deepening the colour, and giving it a transparent look. The feel of the fabric is improved by making it softer and smoother, and its strength and ability to stretch are also improved.”

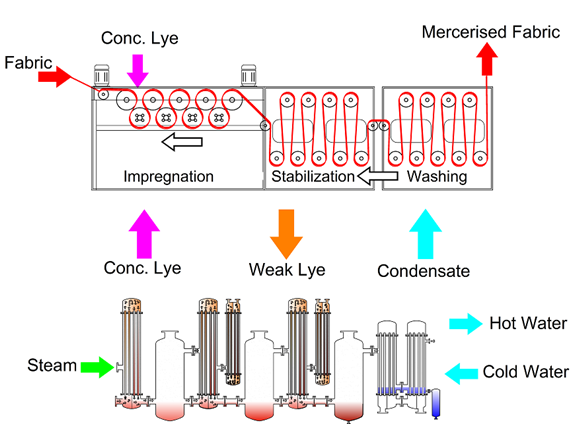
One of two techniques is used to mercerize cotton fibres. The mercerizing process is known as alkaline treatment because the fabric is treated with sodium hydroxide (NaOH), also known as “caustic” solution. There are two types of mercerization with caustic: with and without tension mercerization. Typically, fabrics are subjected to a 270-300 g/L caustic solution under tension for less than one minute. Because the reaction between caustic and fibre is exothermic, the temperature in this popular process is controlled between 5-180 C. Additionally, caustic mercerization can be used at high temperatures. In the realm of cotton mercerization, this process is becoming increasingly popular.
Both of these strategies necessitate stress. There is, however, another method for applying caustic mercerization. This approach employs caustic of a lower concentration (145–190 g/L) and is applied at 20–30°C without tension. Causticizing, caustification, and slack mercerization are all words used to describe this process. There is also a mercerization procedure with ammonia. Cotton yarn or cloth is treated with anhydrous liquid ammonia in ammonia mercerizing. Although the effects are comparable to that of caustic mercerizing, their application is restricted.
Most Important Parameters
In terms of mercerization’s chemical processes, the following parameters are the most crucial:
- Caustic concentration
- Provenance
- Equipment and environmental temperature
- Tension’s
- application duration
The most critical performance determinant parameter of mercerization is caustic concentration. Many variables influence the optimum mercerization bath caustic soda concentration. Concentration, on the other hand, is the most challenging metric to maintain during operation. When it comes to mercerization in the fabric pre-treatment step, for example, the amount of caustic used is critical for dyeing process efficiency. Because caustic is a key component in the mechanism by which fibres absorb colour molecules. Because caustic has a strong affinity for fibres, washing off caustic and dye after mercerization becomes more difficult.
As a result, the concentration of caustic is critical for providing successful dyeing. At normal temperatures, the concentration of the caustic solution shouldn’t be less than 240 Be (210–220 g/L). This will make sure that the mercerization is done completely.
Multistage Evaporation Plant for Caustic Recovery
Based on evaporation from natural circulation, the CRP is formulated. The steam from the boiling water cools and condenses on the outside of the tubes, heating the lye inside. The heating tubes bring the lye to a boil, and the mixture of boiling lye and steam flows into the separator. The separator is set up sideways so that it can separate the steam from the circulating lye. The next step is to heat the vapour to make steam. A partial vapour flow is used to heat up the weak caustic soda.
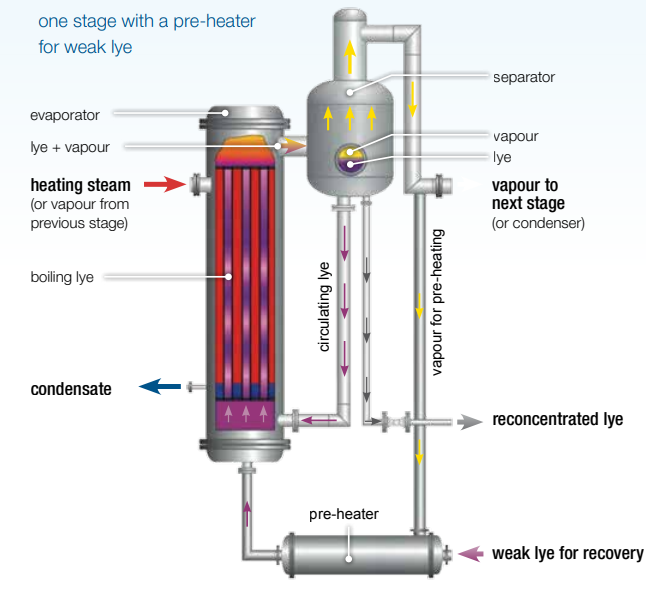
3-stage Caustic Recovery Plant (CRP)
Through a return pipe, the lye that has been separated goes back to the evaporator. With a swirl droplet separator inside the separator, the alkaline liquid can’t get into the vapour phase. The evaporation plant works because of the difference in pressure between the stages. The hardest part is in the beginning. A steam jet vacuum ejector (v) with an after-condenser (ac) or a liquid ring vacuum pump is used to keep the final stage vacuum. In the first stage (1), living steam makes vapour. In the second stage (2), the vapour is turned into heating steam (2). (2). The water that was heated by the steam in the first step goes back to the boiler. The vapour from the second stage heats the third stage (3).
In the condenser, water is used to cool the vapour from the last stage, which in this case is the third stage (c). So, the leftover heat from the stage before is used to heat up the water that has been cooled. The more steps a system has, the less hot steam it needs. Since textile businesses use a lot of hot water, the number of CRP steps needs to be changed in a way that makes sense. Sometimes, a three-stage evaporation plant is cheaper than a four-stage plant with two pre-heaters (p1 and p2), a steam jet vacuum ejector (v), and an after-condenser (ac).

Caustic Recovery from Mercerizing Wastewaters
As previously stated, numerous chemicals and additives are used to process fibres in textile manufacture to improve process efficiency or provide certain properties to the fibres. Alkali, acids, salts, and solvents are the most common chemicals used in textile processing. Membrane filtration, chemical precipitation, activated carbon adsorption, and evaporation are some of the processes utilised to separate these valuable elements from wastewater. The only one of these membrane applications that separates contaminants without the use of chemicals. There are countless instances of environmental and economic applications of chemical recovery and reuse.
Porter conducted research on the recovery of polyvinyl alcohol (PVA) and hot water from discarded textiles. An investigation was conducted into membrane-based methods for recovering PVA, one of the sizing process chemicals used in significant quantities. In addition, it has been emphasised that by recovering PVA, hot process effluent would be reusable due to the cleaning impact of membrane operation.
Schlesinger has investigated the separation of hemicelluloses from viscose fibre production process liquors using a variety of NF and UF membranes. All of these polyether sulfone-based membranes were utilised to evaluate the possibility for recovery. Because the wastewater containing hemicelluloses is extremely alkaline (200 g/L NaOH) and high in temperature, membranes that are resistant to these demanding working conditions were selected for the investigation. Lastly, it is important to highlight that all membranes tested successfully separated hemicelluloses and caustic.
In the textile industry, caustic is one of the most often used chemicals for dyeing and, more significantly, finishing. As previously indicated, caustic should be collected due to harmful environmental effects and financial issues. When caustic use exceeds 11,300 tonnes per year of 100% cotton products or 22,700 tonnes per year of 50/50 cotton polyester fabrics, caustic recovery becomes economically viable. Large textile mills that utilise wet processes, particularly mercerization, are strongly advised to implement caustic recovery.
There are two distinct alkali recovery strategies.
- Recovery of caustic via evaporation
- Recovery of caustic by membrane technology
Evaporation can be used to recover rinsing waters from the caustification process’s washing steps. Caustic in rinse water is not concentrated enough to be reused in the process (40 – 50 g NaOH/L). Weak caustic can be concentrated to reusable levels using a multi-staged evaporation process. It is necessary to purify caustic soda after it has been concentrated. Purification may consist of simple sedimentation or oxidation/flotation with injection of hydrogen peroxide, depending on the method used to recover caustic. A three-stage evaporation process is shown in the diagram below.
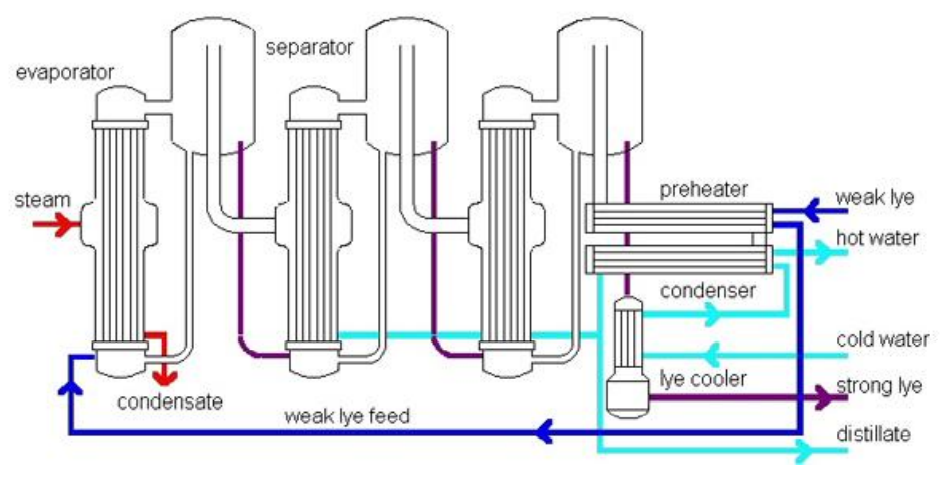
The disadvantage of the evaporation approach is that it cannot recover tainted caustic solutions. Although there are alternative techniques for recovering water and chemicals, membrane filtration allows for the recovery of raw materials by eliminating pollutants without the use of extra chemicals. In the salt and alkali recovery sector, water, sodium hydroxide, and monovalent ions are successfully separated from big organic molecules employing NF systems.
Membrane systems are increasingly being used in textile alkaline recovery applications. The membrane system is fed the same waste stream as the evaporation system. There are several membrane methods that can recover 100% of the caustic. Caustic soda that is pure and concentrated is obtained by membrane filtering. However, the concentration recovered is insufficient to be employed in the procedure again. As a result, membrane systems are typically accompanied with an evaporation unit to raise the caustic soda concentration to reusable levels. Due to the nature of the process and the intense corrosiveness of caustic soda solutions, membrane operation is more problematic. Therefore, membranes that are resistant to caustic recovery should be preferred.
Arrangement of membrane processes in accordance with their driving forces
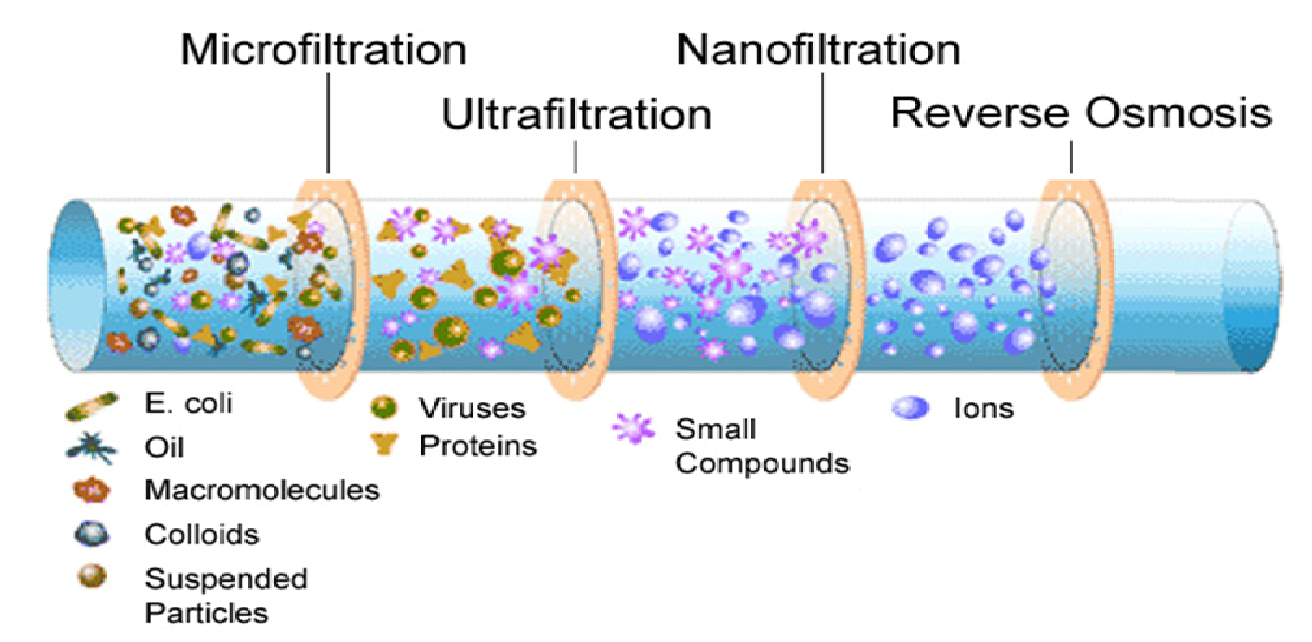
1. Microfiltration (MF)
The MF technique is a low-pressure membrane crossflow separation technology that is used for the separation of colloidal and suspended particles ranging in size from 0.05 to 10 microns. It is like conventional coarse filtration and is commonly used for clarifying. Microfiltration membranes with high porosity and a narrow pore size distribution perform better in terms of process efficiency.
MF membranes are made from a thin polymer film with uniform pore size and a pore density of around 80%, while they may be made from a variety of materials including polymers and inorganic materials. Hydrophobic and hydrophilic synthetic polymeric membranes are the two types of synthetic polymeric membranes. Below are some examples of hydrophobic and hydrophilic membranes. Furthermore, inorganic membranes with excellent chemical and thermal resistance can better manage pore size in MF membranes.
Polymeric Membranes May be Split Into Two Categories.
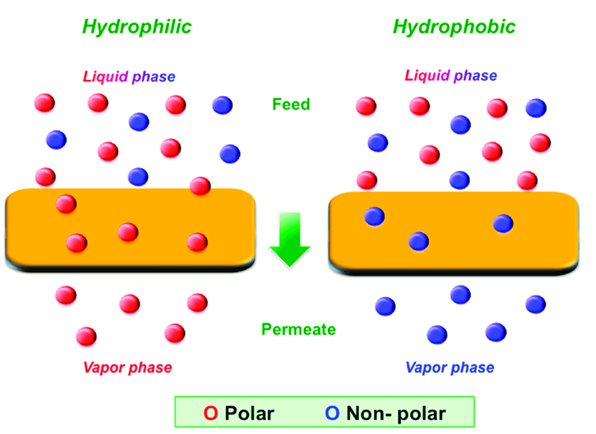
- Membranes made of hydrophobic polymers
Materials like poly(tetrafluoroethylene) (PTFE, Teflon), poly(vinylidene fluoride), poly(propylene), and polyethylene (PE)
- Suitable Polymeric Membranes for Water
Esters of cellulose, The content of polycarbonate (PC), Polysulfon/poly (ether sulfone). Poly (ether imide)/polyimide (PI/PEI), (aliphatic) polyamide (PA), Polyethylene ethyl ketone (PEEK).
In many industrial applications, microfiltration is preferred for big particle (>0.1m) filtration needs. The most prevalent strategy in use today is dead-end operation. Microfiltration systems with crossflow are utilised in large-scale industries.
2. Ultrafiltration (UF)
UF refers to the membrane process between MF and NF. With a membrane pore size ranging from 0.001 to 0.5 m, ultrafiltration is used to separate particles with a low molecular weight from macromolecules. Although MF and UF share the same filtration technique, microfiltration is structurally distinct from ultrafiltration. The density of the top UF membrane layer is much higher than that of the bottom layer. Stronger hydrodynamic resistance is present in this configuration. For UF membranes, polymeric materials are favoured because to their greater microfiltration properties. UF membranes are utilised in a range of industries to separate high-molecular-weight components from low-molecular-weight components. The following are examples of UF membrane components.
- Polysulfonated/Polyether Sulfone/Polysulfon
- Poly (vinylidene fluoride)
- Polymerized acrylonitrile (and related block-copolymers)
- Cellulosic (e.g., cellulose acetate)
- Polyimide/poly (ether imide)
- Aporiferous polyamides
- Polyetheretherketone
Cut-off becomes a critical criterion for choosing an appropriate UF membrane. The molecular weight that the membrane rejects 90% of the time is called the cut-off. However, the cut-off parameter alone is insufficient to identify membrane separation properties. It is essential to analyse the shape and flexibility of solute molecules, as well as their interaction with membrane material. Fouling and concentration polarisation are essential performance indicators for the UF process. As a result, membrane materials’ chemical and thermal resistances become a severe issue. Furthermore, physical factors such as the architecture of the membrane process system have an impact on the fouling occurrence.
Reverse Osmosis and Nanofiltration
The NF and RO procedures are used to extract low molecular solutes from solvents, such as inorganic or tiny organic solutes. Utilized are membranes that are significantly denser and more hydrologically resistant. Additional pressure gradients are necessary for this structure. The pressure range for reverse osmosis is 20 to 100 bars, but the pressure range for NF is 10 to 20 bars. NF is significantly more correlated with process efficiency than MF and UF.
In most cases, the MWCO of NF membranes falls somewhere in the region of 200 to 1000. On the other hand, membranes utilised in reverse osmosis processes have MWCO values that are closer to 100. Regardless, neither semi-permeable membrane has identifiable pores. Despite the fact that they are both pressure-driven processes, reverse osmosis is more efficient in separating dissolved chemicals.
Caustic Recovery with Membrane Technology in the Textile Industry
To clean and prepare textiles for dying, the textile industry makes extensive use of caustic chemicals (sodium hydroxide). Caustic is expensive, and its disposal could provide a concern with hazardous waste. Widely utilised to concentrate and recover diluted, polluted caustic in these processes is evaporation. However, evaporators cannot recover caustic that is severely polluted. It was once required to be neutralised before to disposal. Membrane technology currently permits the recovery of very contaminated caustic. By combining its expertise in polymer research, membrane technology, and process engineering, it provides customised solutions for your most pressing waste reduction, raw material recovery, or liquid processing issues, including caustic recovery.
Benefits Caustic Recovery with Membrane Technology
- Cost savings
- Reduction in caustic waste discharge
- Reduced caustic purchase requirements
- Reduced energy consumption
- Process enhancement
- Reusable caustic after cleaning
- Enhanced evaporator performance
- Reduced condenser blowdown
- savings in waste management
How the System Functions
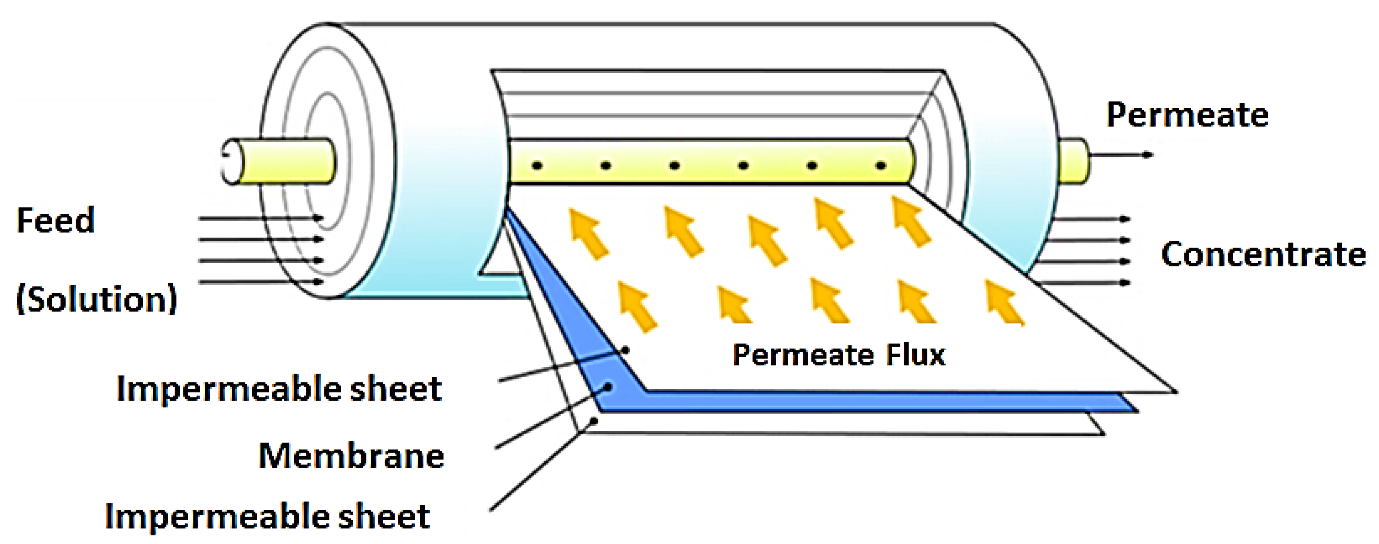
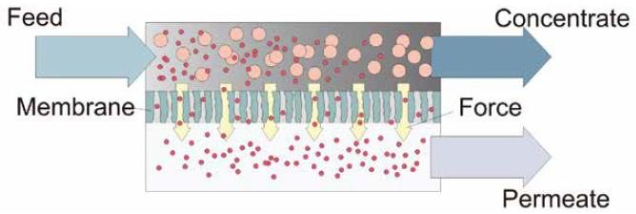
The polluted caustic feed stream is supplied into one end of a porous, membrane-lined stainless-steel tube. Clean caustic and water will pass through the membrane-lined porous tube’s wall and be collected for reuse. At the opposite end of the tubing, the concentrated contaminants exit for treatment or disposal.
Membrane Processes
Membrane techniques have been used to treat textile effluent for a long time. They’ve shown that they can handle the fluctuating and heavily contaminated textile sector effluents. In addition, membrane processes are utilised in the textile sector for the purpose of recovery. Membrane systems are utilised for numerous chemical and water recoveries, including size, caustic, and indigo dye.

It is vital to select the appropriate membrane system and technology for the specific wastewater. However, previous research on the textile industry indicates that a pre-treatment method is typically employed prior to the membrane unit. Pre-treatment is also necessary and more efficient than chemical cleaning in terms of preventing and retaining fouling mechanisms in subsequent membrane operations. The membrane’s performance is improved, and operational costs are reduced, thanks to pre-treatment. Membrane fouling is unavoidable if pre-treatment is not used, especially due to the high particle’s concentration of textile wastewater. Pre-treatment is therefore required to prevent membrane fouling and degradation.
Membrane is a type of equipment used to selectively separate two phases or substances. Membranes are a relatively recent separation technology, yet they have a wide range of applications and are improving all the time. There are a variety of membrane configurations accessible today, including those that are homogeneous or heterogeneous, symmetric, or asymmetric, solid or liquid, and positively, negatively, or neutrally charged. Many conditions, including pressure, temperature gradient, electrical field, and concentration, may alter the transport phenomenon within these membranes, given this discrepancy.
Water resources are scarcer and more valuable today. This allows the companies that consume the rawest water to make better use of their resources. According to current research, treating wastewater created by operations for reuse rather than dumping it into the waste stream is the best alternative for industries. For reuse and recovery, pressure-driven membrane methods are more fulfilling than traditional procedures.
The Fundamentals of Membrane Processes
Membrane processes can be viewed as the separation of a stream into permeate (the stream component that can pass through a semipermeable membrane) and retentate (the portion of the stream that is rejected). For this separation process, a driving force is necessary.
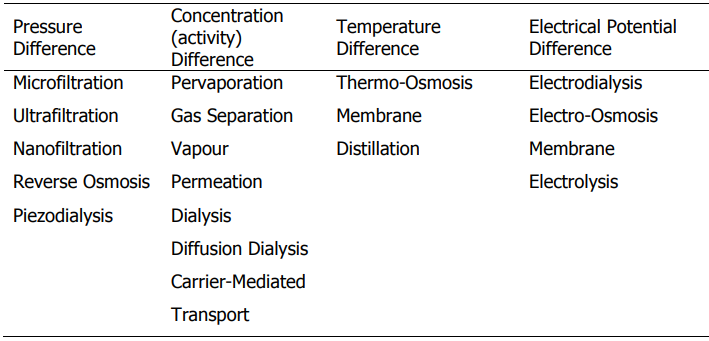
It could be a variation in the system’s pressure, concentration, or temperature. This force affects the performance of the process to some degree. In addition, the features of the membranes play a significant effect in the efficiency of the process. The sort of process that is being carried out may be largely inferred from either the structure or the composition of the membrane. When charged particles need to be transported across a membrane under certain circumstances, the difference in electrical potential can act as a driving force in such cases.
The table that follows provides an illustration of the categorization of various membrane processes according to their respective driving factors.
Recovery of Caustic Soda in Textile Frequently Asked Questions
1) Why caustic soda NaOH is used in textile industry?
The process of making and using caustic soda, also known as sodium hydroxide or lye, began with the manufacture of soap. It is now widely utilised in the textile industry for operations including scouring, mercerization, and dyeing, crucial steps in the production of textiles.
2) How do you recover caustic soda?
After the addition of a silica source in the form of zeolite A, a micro porous substance that is widely utilized in many technological fields, caustic soda was regenerated at a rate of around 70% by precipitating aluminate.
3) What is the role of caustic soda in the industrial cleaning process?
Due to its capacity to dissolve proteins, fats, oils, and grease, caustic soda plays a crucial part in eliminating stains from hands and commercial textiles.
4) Which process leads to the formation of NaOH?
It is processed with water in the denuder to create a sodium hydroxide solution. As the fluid evaporates, sodium hydroxide solidifies. This method is quite effective for getting pure caustic soda.
5) What are the benefits Caustic Recovery with Membrane Technology?
- Cost savings
- Reduction in caustic waste discharge
- Process enhancement
- Reusable caustic after cleaning
- Enhanced evaporator performance
- Reduced condenser blow down
- Reduced caustic purchase requirements
- Reduced energy consumption
- Savings in waste management