Pressure Driven Membrane-ion Exchange Process
Overview:
A new technology that is being experimented to treat water to attain the utmost purity is a combination of ion exchange resin and pressure driven membrane process. This is a hybrid process in water treatment technology and is mainly used because it is cost effective, produces high quality purified water and optimizes the treatment process. As stated in literature, the hybrid ion exchange and pressure driven membrane process s used to prevent the fouling of membrane during the process of water treatment, desalination of sea and brackish water and removal of targeted pollutants. The efficiency of membrane is highly effected due to fouling by organic compounds. Even though organic pollutants can be pre-treated and removed by coagulation and flocculation but in most cases this pre-treatment is not sufficient. Especially dissolved organic matter cannot be removed by coagulation and flocculation. Therefore, it is recommended to used hybrid ion exchange and pressure driven membrane process to thoroughly remove natural and dissolved organic matter.

What does it remove?
This hybrid process is mostly used to remove trace metals from water like boron, heavy metal and oxyanions.
Removal of boron:
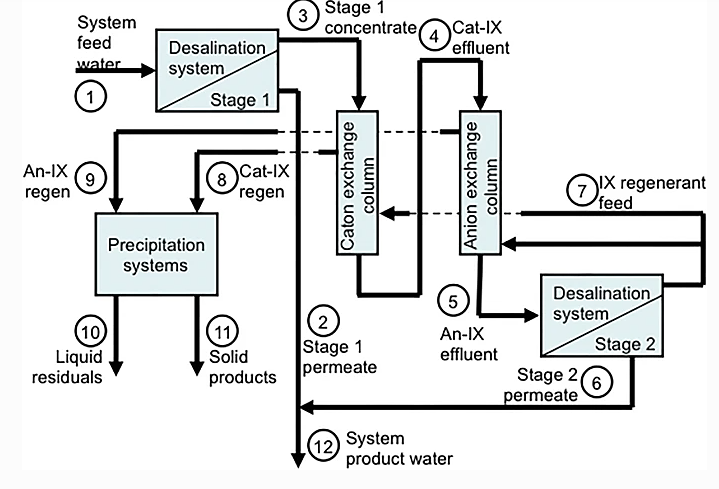
Ion exchange and pressure driven membrane hybrid process can very efficiently remove boron from waste water. if the amount of boron present in water is below 0.3mg/L then it is beneficial to both animals and humans, however if the concentration increases beyond that, it becomes hazardous to all life forms. In most areas such as Africa reverse osmosis is employed to remove boron from seawater. Seawater is mostly purified in Africa to use as drinking water and for irrigation. However, reverse osmosis is not as efficient because seawater contain 5mg/L and reverse osmosis can only remove 1-2mg/L of boron from seawater, which is till harmful for consumption. However, post treatment of RO permeate with boron selective chelating resins can very efficiently remove the remaining boron. Commercial boron selective chelating resins are microporous crosslinked polysteric resin, functionalized with N-methyl-D-glucamine groups. Covalent attachment and formation of an internal coordination complex facilitates the capture of boron by these groups. Only boric acid is removed from the feed as boron selective chelating resins are highly selective and does not affect the concentration of other ions. The use of Boron selective chelating resin to remove boron from RO permeate can also reduce capital and operational costs. Because in conventional RO system in order to efficiently remove boron a second RO pass is required which increases energy consumption, moreover, to provide high rejection of ionized boric acid the RO pass will have to operate at a higher pH, this in turn requires the use of caustic soda and antiscalant. The use of an RO pass will also increase the size of the RO system. All of these would increase the operational costs and will make the process more complicated. Moreover the purity of water obtained will be less as compared to when boron selective resin is used.

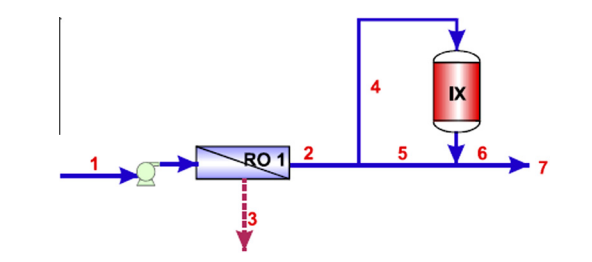
Table 1: Performance comparison between the Double Pass RO and the hybrid RO-IXR Systems for Boron removal from Seawater
| Double Pass RO | Hybrid RO-IRX | |
| RO 1st pass size (m3 /d) | 121,800 | 111,900 |
| RO 2nd pass size (m3 /d) | 48,250 | NA |
| IXR size (m3 /d) | NA | 37,270 |
| Total waste (m3 /d) | 67,000 | 61,340 |
| TDS of final product (mg/L) | 79 | 480 |
| Boron content of final product (mg/) | 0.48 | 0.47 |
| By-pass (%) | 12 | 26 |
Benefits:
- Low operational costs
- More flexible
- Savings of membrane area
- Reduction in total waste
- Increase in by pass volume
- Highly purified water
Hybrid ion exchange- membrane processes for wastewater treatment
Large amount of flow back water is produced due to shale gas production. This flow back water can be treated by using a hybrid process consisting of ceramic membranes filtration and ion exchange resins. Ceramic membranes of 1.4 µm and 0.2 µm of pore size and mixed bed IXR are used. Because pf small footprint both membranes and IXR stages can be used for on-site treatment. The water first goes through the membrane treatment stage and total suspended solids and turbidity are removed. The water goes through the IXR stage to remove total dissolves solids. All of total suspended solids and >99% of total dissolved solids can be removed by this process.

Table 2: Composition of raw flowback water and composition water after hybrid MF-IX treatment.
| Constituent | Raw flowback water | After Treatment |
| TOC (mg/L) | 720 | 82 |
| TSS (mg/L) | 881 | 0 |
| pH | 6.85 | 6.91 |
| Conductivity (µs/cm) | 67,000 | 54 |
| Na (mg/L) | 12,200 | 3.7 |
| K (mg/L) | 363 | <0.5 |
| Mg (mg/L) | 104 | <0.05 |
| Ca (mg/L) | 2935 | <0.1 |
| Ba (mg/L) | 697 | <0.05 |
| Sr (mg/L) | 591 | <0.05 |
| Al (mg/L) | 105 | <0.05 |
| Fe (mg/L) | <1 | <1 |
| Mn (mg/L) | <2 | <0.05 |
| Cl (mg/L) | 28,500 | 7.5 |
| Br (mg/L) | 19 | <0.01 |
| F (mg/L) | <1 | <0.01 |
| SO4 (mg/L) | 12.9 | <0.05 |
Treatment and desalination of brackish/seawater using hybrid IX-RO/NF processes
Desalination of brackish water by hybrid IX-RO/NF process reduces cost and prevents membrane fouling. Most commonly reverse osmosis is used for the desalination of brackish water, but its drawback is that scaling of membrane takes place due to which the efficiency of the process is reduced and water treated is not highly pure. Pre-treating water with antiscalants before sending it to RO membranes can minimize scaling but won’t completely remove it. However, IX-Ro/NF process prevents scaling and produces highly purified water.
Advantages
- Cost effective.
- Produce high quality purified water.
- Flexible process.
- Better performance of water treatment processes.
- Prevents scaling of membranes.
- Removes only the targeted pollutants.
- Uses less energy and space.
- Consistent water quality.
- Enhance filtration performance.
- Reduction in discharge amount.
Limitations:
- Resin regeneration cost.
- Process configuration
- Holistic feasibility of hybrid technology.
Ion Exchange Process Frequently Asked Questions
1) What is pressure-driven membrane process?
A pressure applied to the solution at one side of the membrane acts as a driving force to separate it into a permeate and a retentive in pressure-driven membrane processes (reverse osmosis, Nano filtration, ultrafiltration, and microfiltration).
2) Which two procedures are pressure-driven membrane operations?
In decreasing order of pore size, Microfiltration, Ultrafiltration, Nanofiltration, and Reverse Osmosis are the four primary pressure-driven membrane filtration techniques.
3) How are ion-exchange membranes made?
Homogeneous ion-exchange membranes are now made by either inserting anionic or cationic moieties into a polymer that may be in a suitable solution or a premade film, or by polymerizing monomers that carry these moieties.
4) What is the mechanism of ion exchange process?
Ion exchange is a chemical reaction in which distinct ions with similar charges in solution are exchanged for free mobile ions of a solid, the ion exchanger. The exchanger needs to have an open network structure that allows ions to travel through it, whether it be organic or inorganic.
5) What is the principle of ion exchange method?
The process of ion exchange involves transforming the ions in a solution into a solid, which then releases new ions of a different type but the same polarity. This implies that different ions that were once present in the solid take the place of the ions in solutions.
6) How is reverse osmosis used for brackish water?
From the side with the highest salt concentration to the side with the lowest salt concentration, the solvent (water) travels through the membrane. As a result, the portion involving the concentrated solution is reduced in favour of the freshwater.