Crystallizer for Effluent Recycling – Zero Liquid Discharge System
What is crystallization OR Crystallizer?
Crystallization is a physical change. Crystallization is the formation of solids from the liquid or gaseous phase. This technique includes obtaining the crystals of a soluble substance from a hot saturated solution and separating the soluble solid from the solution.
To concentrate feed into solid crystals and clean water, crystallizers are used. A progressively harder method for which crystallite sizes are formed from a liquid solution is known as crystallization. Crystallizers can remove liquid wastes completely, resulting in no liquid discharge (ZLD). The process continues and secondary crystallization are the two stages of crystallization. The formation of new crystals is referred to as primary nucleation. Secondary nucleation is the primary stage that results in the mass production of crystals. There are two types of crystallization processes: evaporative crystallization and cooling crystallization.
The selection of crystallizers necessitates a thorough examination of the application’s requirements. A salt crystallizer, for example, converts brine into solid salt crystals and clean water. Other types of salts are crystallized using a continuous cooling crystallizer (CCC).
Types of crystallization
There are three types of crystallization based on its formation.
1) Evaporation crystallization
It occurs as a result of the evaporation of the solvent. That used to form crystals from inorganic salts and sucrose etc. Therefore, this process results in the suspension of vapors and crystals in the mother liquor.
2) Cooling Crystallization
In this, the liquid to be crystallized is cool down to a temperature lower than the equilibrium solubility, generally the solubility of the liquid increases with increasing temperature. In such cases, cooling of crystallization is generally more energy-friendly than evaporation crystallization.
3) Precipitation
In this type, the crystals are formed from the solution when it reacts. This crystallization process is faster than other types of crystallization because the reaction process is fast. The rapid conversion phase represents the shortest residence time in all precipitation processes.
Crystallizer for Effluent Recycling
Crystallization is the method of separation. In the process of separation, crystals are produced in the form of liquid or gases. The solid crystals produced by the crystallization process are very pure. That is why the crystallization process can also be used as an industrial cleaning process. The crystallization process is not easy and the most important step in the formation of solid crystals in a liquid solution, the solution is concentrated and cooled until the solute concentration is higher than dissolved at that temperature and the solute forms pure crystals.
Crystallizer
Crystallizers are the best technology for installing ZLDs or Zero Liquid Discharge Systems. It converts wastewater into two streams, one containing solid waste with useful resources and the other with high-quality, recyclable water. The function of the vacuum crystallizer is being designed to evaporate all solvents and leave a highly concentrated, pasty, gelatinous, or shiny product. As one of the best ZLD suppliers, this technology in zero discharge system is very efficient and has a very low management cost.
Methods of Supersaturation
For the crystallization process to make happen, the solution must be supersaturated. For crystallization, the method of supersaturation is entirely different from each other. It can be done in three ways:
- The supersaturation filling process can be achieved by cooling the solution but with negligible or slight evaporation.
- Supersaturation can also be produced by solvent evaporation using a little cooling.
- The process of producing supersaturating can be achieved through a combination of cooling and adiabatic evaporation. This process is also known as vacuum crystallization.
To meet the strict requirements for extracting zero liquid it also requires a crystallizer, to reduce the concentration of the brine into a solid dry. The water can be recycled back to the plant processes, while the solids that are easy to handle can be safely disposed of in an approved waste disposal facility.
Types of Crystallizers
1) Forced circulation crystallizer
The system of vapor pressure is often applied to forced circulated crystallizers, which may have one or more effects. They usually run in the width of a low vacuum to atmospheric pressure. Typically, these units are used when crystal size is not important or when the crystal is growing at the right speed. Depending on the application, almost all building materials may be used in the manufacture of these crystallizers.
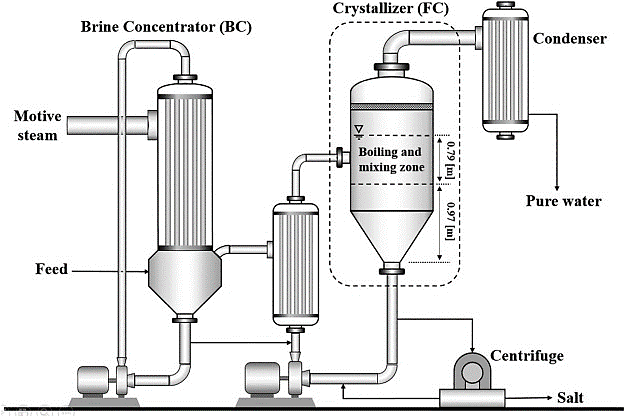
2) Evaporative Crystallizer
Evaporative crystals are used in the process of evaporative crystallization. Through this crystallizer, the solvent evaporates leading to an increase in the concentration of the solution. Due to the increase in concentration, supersaturation of the solution occurs and the nucleation process begins. As nucleation progresses, nuclei grow and it changes the crystals.

3) Oslo Crystallizer
The process of Oslo crystallizers is also known as wet bedding. In the Oslo crystallization process, under the Oslo crystallizer, crystals dissolve or can be converted into a liquid form from a solid form. The fluidization process takes place with the help of an external axial pump. This pump contains the mother liquor in the upper part. This crystallizer includes the central tube blocking with the help of the precipitation process and supersaturation without crystals.

4) Vacuum Crystallizer
The process of vacuum crystallizers is sometimes used as a continuous and sometimes as a collection. In industry, batch vacuum crystallizers are more popular than continuous vacuum crystallizers. Batch vacuum crystallizers are used for the distillation process. Distilled material grows on the crystallization machine in continuous crystallization walls. Vacuum crystals are used to store vacuum inside the crystallizer body with the help of a condenser booster.

5) Cooling Crystallizer
Cooling crystallization is done with the help of indirect heat transfer and under a vacuum. The crystallization cooling process is based on the dependence of the solubility temperature. Through the cooling crystallization process, some of the solutions evaporate and then cool down. Cooling down of the solution occurs with a decrease in pressure in the balance. As a result, a small solvent shines at a hidden temperature following a decrease in the temperature of the solution.
How does a Crystallizer work?
The crystallizers are designed to handle the continuous crystallization of different dissolved salts that can sometimes be recycled as valuable products. Wastewater coatings are used to concentrate impurities in wastewater containers, and when fitted with solid waste removal materials, they incorporate a real zero-fluid drainage system. These brine crystals are usually driven by steam but in some cases can use other technology to recycle vapour to reduce energy consumption and operating costs.
Crystallization is done by evaporation, where all water evaporates and collects to fulfil the reuse purposes. The addition of acid helps to create a solution so that, when it heats, it can avoid measuring and damaging the heat exchangers. DE aeration is commonly used in it simply to dissolve dissolved oxygen, carbon dioxide, and other unconventional gases. The remaining waste from the evaporator to the crystallizer continues to boil all the water until the impurities in the water begin to form crystals and are filtered as solid in ZLD system.
In this process, the solutes must have a melting curve that will be able to decrease in temperature if the crystallization process uses supersaturation by cooling. The process of supersaturation is achieved by evaporation of solvent only where the solubility depends on the temperature. Crystallization occurs in an evaporator/crystallizer that is forced to rotate, where it generates and add crystals within the mass solution. The crystallizer system is followed by a water extraction device, which separates the salt crystals from the product slurry. The mother liquor is returned to the crystallizer for further concentration.
The evaporator circulation is usually supplied with an external steam heating source due to the high boiling point of the solution at high concentrations. The process of vacuum crystallization involves a combination of evaporation and cooling when the solution is placed under vacuum conditions, as a result of which the solvent suddenly evaporates and cools the solution through an adiabatic process.
There is usually a side opening to facilitate access to the manual extraction of shiny salts. At the end of the cycle, the vacuum is released and the contents of the boiler are removed manually with a scraper blade through one of the front doors. Crystallizers can work well with heat pumps, steam, or hot water. This approach is one of the most common ways of achieving greater industrial efficiency and this process also depends on the properties and characteristics. The crystallization process is not an easy one and is the most important part of producing solid crystals from liquid form. First, the solution is concentrated and later reduced, until the solute concentration is higher than dissolved. As a result, the solute will form pure crystals. The speed at which crystals are formed is known as the crystallization rate.

Applications of Zero Liquid Discharge System
- The process of crystallization is used in various applications of industries. The industrial use of crystallization is not always obtained from pure crystals. Often the crystallization process is used for the extensive treatment of liquid waste in a river or ocean.
- It is useful when treated wastewater cannot be wasted.
- The amount of waste filling when external management costs are very high.
- To treat contaminated water when conventional methods do not work.
- Cleaning of compressors, and water from the ground.
- Focus on materials.
- Work baths and showers with galvanic procedures and local treatments.
Advantages of Zero Liquid Discharge System
- Reduce the amount of waste to be managed.
- Significant reduction in waste management costs.
- Encouraging the reuse of an important part of the liquid.
- The probability of implementing a zero-discharge system.
- Fulfilment of current regulations regarding waste disposal.
- Reducing the need to store more waste.
- Reduce greenhouse gas emissions when transporting waste.
- Reducing the use of piped water by re-using clean water.
- Lack of reagents.
Environmental Features
- ZLDs consume a large amount of energy, but this may be improved by emerging technologies (solar or geothermal energy) and enable further reduced emissions of GHGs.
- Disposal of solid waste in landfills can leach chemicals into groundwater and may cause groundwater pollution.
- Some pre-treatment processes, such as acidification followed by degasification release an amount of CO2 into the atmosphere.