Advancements in Fenton Process for Wastewater Treatment
Introduction
H.J.H. Fenton identified Fenton oxidation in 1894. The Fenton process’ oxidation process has been investigated for about 90 years. According to studies, the Fenton process involves upwards of 20 chemical reactions, with the core reaction being the most well-known. Fenton’s reagent is a hydrogen peroxide (H2O2) solution containing ferrous iron (usually iron (II) sulphate, FeSO4) as a catalyst for oxidizing pollutants or effluent fluids. Organic substances including tetrachloroethylene (TCE) and trichloroethylene (TCE) can be destroyed using Fenton’s reagent. Most persistent organic contaminants can be promptly and non-selectively degraded to carbon dioxide and water by the extremely oxidative hydroxyl radical (*OH) produced by the interaction of H2O2 with Fe2+ in strong acid. The Fenton process’ oxidation mechanism is depicted below.

The Fenton method involves three major flaws:
- a narrow pH range
- high costs and risks connected with handling, transportation, and preservation of reagents
- large iron sludge-related secondary pollution
The Fenton process is constantly optimised and enhanced in order to solve three flaws, resulting in a variety of optimised Fenton procedures. As a result, the fundamental concepts and applications of several optimised Fenton processes for organic treatment of wastewater were evaluated from a needed skill, using the Fenton process’s inadequacies as a breakthrough. In several optimised Fenton processes, some essential operation parameters such as effluent pH as well as catalyst, H2O2, and organic pollutants content were explored in detail. Suggestions for future research for improved Fenton processes are proposed below.
Processes of Single Fenton Optimization
1. Heterogeneous Fenton Method
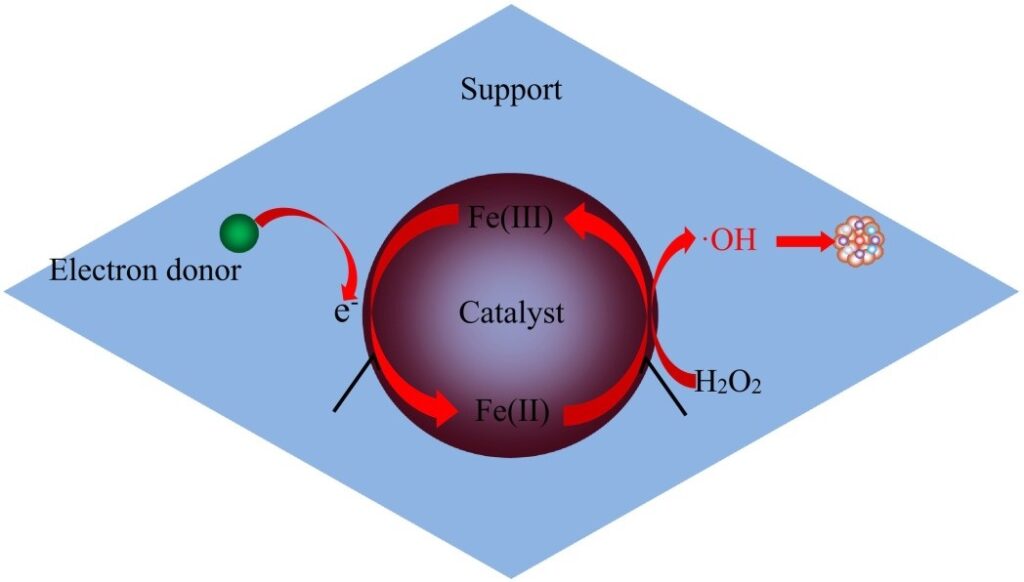
The heterogeneous Fenton method has been extensively explored in order to solve the shortcomings of the standard Fenton process, which is restricted to a narrow operating pH range and creates a massive portion of iron sludge. In the heterogeneous Fenton system, catalyst Fe2+ is modified by a solid catalyst comprising catalytic active elements, allowing Fenton catalytic reaction to happen at the active site here on surface of solids catalyst, attempting to prevent iron ions from broadening the operating pH range, leaching, and lowering iron sludge production.
The findings reveal that iron ions leaking with in heterogeneous Fenton system is exceedingly low, with levels far below the permissible limit of 2 mg/L set by European Union directives. The following are the key factors:
- The hydrothermal approach as well as other configuration parameters is used to immobilize iron entities with catalytic activity on various substrates like zeolite, graphene, clay, and activated carbon. The protective actions of supporting prohibit iron species from becoming immersed in the solution, reducing iron species loss.
- The heterogeneous Fenton catalyst’s composition and structure enable electron transport from of the electron donor to Fe3+, speeding the decrease of Fe3+ to Fe2+, and ensuring effective Fe3+ and Fe2+ cycling upon that heterogeneous Fenton catalyst.
The secret to a good heterogeneous Fenton reaction is the development of protracted stabilized heterogeneous Fenton catalysts having high reactivity that can be employed over a wide pH range and easily removed without using additional energy. Supporting and non-supporting Fenton catalysts are the most common heterogeneous Fenton catalysts today. To tackle the problem of iron leaching, supported catalysts immobilize iron compounds on different supports. By depositing Fe2+ on strong magnetic porous carbon microspheres, Zhou et al. (2014) created an effective heterogeneous Fenton catalyst. The heterogeneous Fenton technique was utilised to extract methylene blue (MB) from contaminated water using the effective heterogeneous Fenton catalyst that had been produced. The analysis indicates that the rate of degradation of MB was greater than 90% within 40 minutes at pH 6.18, indicating that the catalysts had significant catalytic performance for the breakdown of organics at neutral pH. Ma et al. (2018) used microalgae to make a new Fe-N-graphene wrapped Al2O3/pentlandite compound. The Fenton catalytic capability of the composite was high, owing to the synergistic actions of Fe, Al, and Ni. Furthermore, the catalyst was reused 12 times and essentially maintained the same catalytic activity, indicating that catalyst exhibited exceptional long-term durability. Natural iron-containing minerals are the most common non-supported catalysts, which can directly form a heterogeneous Fenton system using H2O2 to breakdown refractory organic contaminants. Z.X. Liu et al. (2018) suggested a template technique strategy for synthesizing ultra-small -FeOOH nanorods (SFNs) as well as studied its usage for accelerating the breakdown of methyl orange, an azo dye (MO). The increased surface area and much more catalytic spots of SFNs may explain the increase MO degradation rate of 98% were observed.
2. Photo-Fenton technique
Because of the low iron concentrated sludge, the Photo-Fenton process has received a lot of attention to the reduction of recalcitrant organic contaminants. The use of ultraviolet and visible light in conjunction with the traditional Fenton process can improve catalyst functional capacity, enhance organic pollutant degradation rate, and reduce iron sludge generation. The goal of the photo-Fenton method is to use visible light to expedite the decrease of Fe3+ to Fe2+. At pH 2.8–3.5, Fe2+ interacts quickly with H2O2 to produce Fe3+, which is mostly found as [Fe(OH)]2+. [Fe(OH)]2+ experiences metal charge transport stimulation when exposed to light, regenerating Fe2+, which catalyses the disintegration of H2O2 and produces extra radical *OH, which destroys organic contaminants.
![]()
Furthermore, immediate photolysis of H2O2 generates the radical dot OH, which can be employed to degrade organic contaminants.
![]()
As a result of the synergistic catalytic actions of Fe2+ and light, more radical *OH can be produced, increasing oxidation effectiveness in the photo-Fenton method.
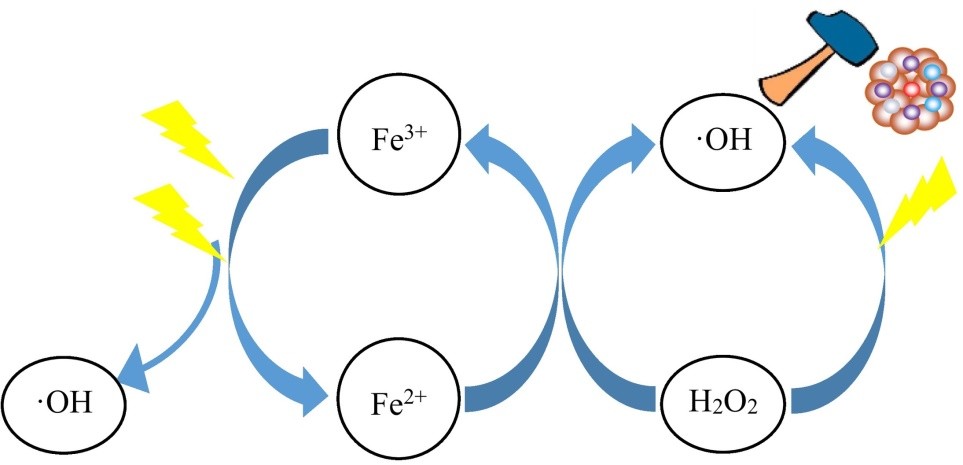
The photo-Fenton process relies on a light irradiation mechanism that has a considerable effect on the rate of organic pollutant destruction. According to research published in the literature, UV light and sunshine is the most conventional light supplies in the photo-Fenton reaction.
3. The Electro-Fenton method
The Electro-Fenton process was created to address the drawbacks of the traditional Fenton process, such as the formation of iron sludge as well as the significant expense involved and risk with reagent management, transport, and storing. Because of its features such as adaptability, lots of energy economy, automated capability, and environmental friendliness, electrochemistry can significantly improve the traditional Fenton process. As a result, the electro-Fenton method is created by combining the traditional Fenton process with electrochemistry. The basic idea is that H2O2 is produced in situ by electrochemically reducing O2 upon that cathode, avoiding the expenses and risks of processing, transport, and keeping H2O2. As well as the Fe3+ produced by the Fenton reaction is converted to Fe2+ upon that cathode, allowing for Fe2+ regeneration and a reduction in iron activated sludge. The electro-Fenton process is divided into four categories based on the addition or creation of Fenton reagents:
- cathode electro-Fenton process
- sacrificial anode electro-Fenton process
- Fe2+ cycling electro-Fenton process
- Fe2+ cycling electro-Fenton process
Fe2+ is supplied externally to the EF-H2O2 process, whereas H2O2 is created in situ by the catalytic decomposition of O2 on the cathode.
![]()
This can save money and time by avoiding the expenses and risks of storing, transporting, and storing H2O2. H2O2 is supplied independently to the EF-Feox process, whereas Fe2+ is electro-generated via a sacrificial anode. This can eliminate necessity of Fe2+
![]()
The EF-Feox method, on the other hand, has significant drawbacks, such as a high anode consumption rate and a substantial portion of iron sludge generation. H2O2 and Fe2+ are both injected externally in the EF-Fere process, however Fe3+ formed by the Fenton reaction is converted to Fe2+ upon this cathode, reducing iron sludge generation and the original Fe2+ content input.
![]()
The EF-H2O2-Fere process combines the EF-H2O2 and the EF-Fere processes. H2O2 is created in situ via cathodic decrease of O2 and Fe2+ is renewed via elimination of Fe3+ onto the cathode in the EF-H2O2-Fere process, which eliminates the need for H2O2 and reduces iron sludge formation and original Fe2+ concentration input. The constant in-situ electro-generation producing H2O2 plays a major role in the electro-Fenton method and is influenced by the kinds and qualities of the cathode, according to the electro-Fenton program’s basic principle.

Fenton Process Frequently Asked Questions
1) What is Fenton reaction in wastewater treatment?
The most popular AOP for wastewater treatment is Fenton’s reaction, which has promising potential for removing PPCP from wastewater and water bodies. Fenton’s reaction has advantages in terms of reaction speed, chemical consumption, and ease of hydroxyl radical production.
2) What is advanced oxidation process in wastewater treatment?
Advanced oxidation processes (AOPs) are wastewater treatment techniques designed to break down and mineralize organic debris that is resistant to degradation through interaction with the hydroxyl radical (•OH).
3) What is heterogeneous Fenton process?
Fenton-like heterogeneous reaction When Fe+2 is either swapped out for Fe+3 or other transition metal ions in the Fenton reagent system, a Fenton-like reaction is established (Wang et al. 2016).
4) What is photo Fenton process?
For the treatment of industrial wastewaters, the photo-Fenton technique is currently regarded as a common advanced oxidation process (AOP). To speed up the conversion of Fe3+ to Fe2+, this photochemical technique uses UV radiation.
5) Why is the Fenton reaction important?
The conversion of weakly reactive radicals into highly reactive ones is largely mediated by the superoxide-driven Fenton reaction.