IoT and AI in Pharmaceutical Manufacturing
1. Introduction
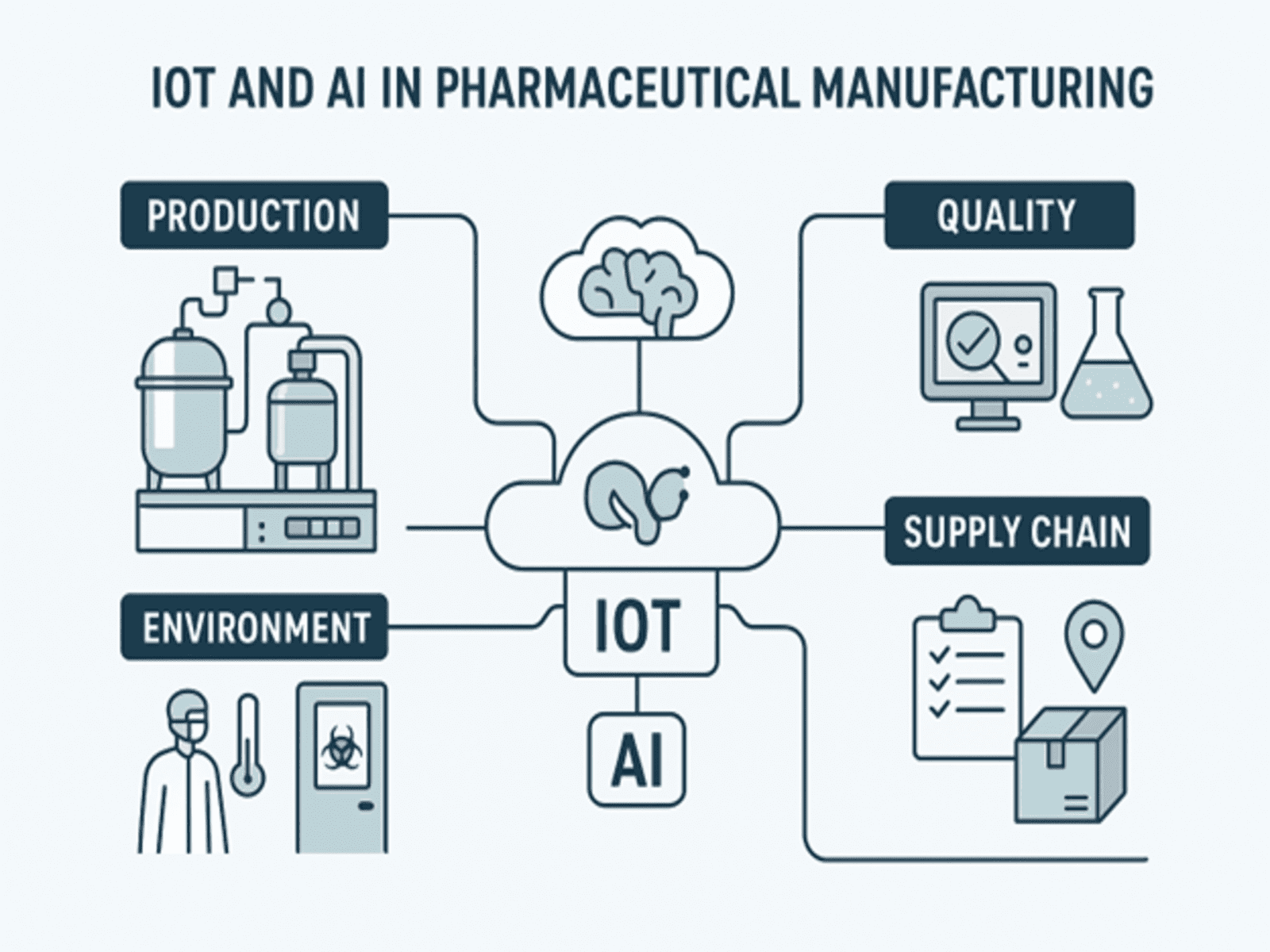
Pharmaceutical manufacturing is one of the most regulated industries, requiring precision, consistency, and compliance with Good Manufacturing Practices (GMP). Traditional processes are labor-intensive, with limited real-time monitoring and reactive maintenance. By integrating Internet of Things (IoT) and Artificial Intelligence (AI), pharma companies can move toward Industry 4.0 — achieving real-time monitoring, predictive insights, automation, and data-driven decision-making.
2. Role of IoT in Pharmaceutical Manufacturing
IoT connects physical equipment, sensors, and processes into a digital network. In pharma, IoT applications include:
- Environmental Monitoring
- Sensors monitor cleanroom air quality, temperature, humidity, and pressure differentials.
- Ensures compliance with GMP and FDA 21 CFR Part 11 regulations.
- Equipment & Process Monitoring
- IoT sensors track machine vibration, temperature, and pressure in reactors, centrifuges, tablet presses, and packaging lines.
- Prevents unplanned downtime through real-time alerts.
- Supply Chain Tracking
- RFID tags, GPS, and IoT sensors monitor the movement of raw materials and finished drugs.
- Cold chain monitoring ensures vaccines and biologics remain at the correct temperature during transportation.
- Digital Batch Records
- IoT-enabled devices collect data directly into electronic batch records (EBR), reducing human error.
3. Role of AI in Pharmaceutical Manufacturing
AI processes the vast data streams from IoT devices to predict, optimize, and automate decisions. Key applications:
- Predictive Maintenance
- Machine learning models analyze IoT data (vibration, temperature, noise) to predict equipment failure before it happens.
- Reduces downtime and maintenance costs.
- Process Optimization
- AI models simulate chemical reactions, mixing, drying, and coating processes to optimize yields and reduce waste.
- Adaptive control adjusts parameters in real time (flow rate, temperature, pH, mixing speed).
- Quality Assurance (QA)
- Computer vision powered by AI inspects tablets, capsules, and vials for defects in shape, size, or labeling.
- AI detects anomalies faster than human inspectors.
- Regulatory Compliance
- AI-driven audit trails and anomaly detection ensure compliance with GMP, FDA, and EMA regulations.
- Drug Production Forecasting
- AI predicts demand and optimizes production scheduling.
- Reduces overproduction and inventory waste.
4. Benefits of IoT + AI Integration in Pharma Manufacturing
- Enhanced Product Quality – Consistent drug potency and purity through real-time monitoring.
- Reduced Downtime – Predictive maintenance minimizes production stoppages.
- Regulatory Compliance – Automated data logging ensures audit readiness.
- Operational Efficiency – Optimized energy, water, and material usage.
- Improved Patient Safety – Ensures drugs meet strict safety and quality standards.
- Supply Chain Resilience – IoT-enabled traceability reduces risks of counterfeiting.
5. Example Workflow in a Smart Pharma Plant
- IoT sensors collect data on equipment health, environmental conditions, and production variables.
- Data is sent securely to the cloud for analysis.
- AI models detect anomalies, optimize process parameters, and predict equipment failures.
- Operators receive actionable insights via dashboards.
- Automated control systems adjust operations in real time (e.g., adjusting mixer speed or temperature).
6. Future Outlook
The future of IoT and AI in pharma includes:
- Digital Twins – Virtual replicas of production plants to simulate and optimize processes.
- Autonomous Manufacturing – AI-driven plants that run with minimal human intervention.
- Blockchain Integration – Ensures tamper-proof supply chain records.
- Personalized Medicine Production – IoT + AI will enable flexible, small-batch manufacturing for patient-specific treatments.
✨ In summary:
- IoT acts as the “nervous system” by collecting real-time data from every part of the manufacturing ecosystem.
- AI acts as the “brain” by interpreting that data to optimize efficiency, ensure quality, and maintain compliance.