Arsenic Removal from Drinking and Industrial Water
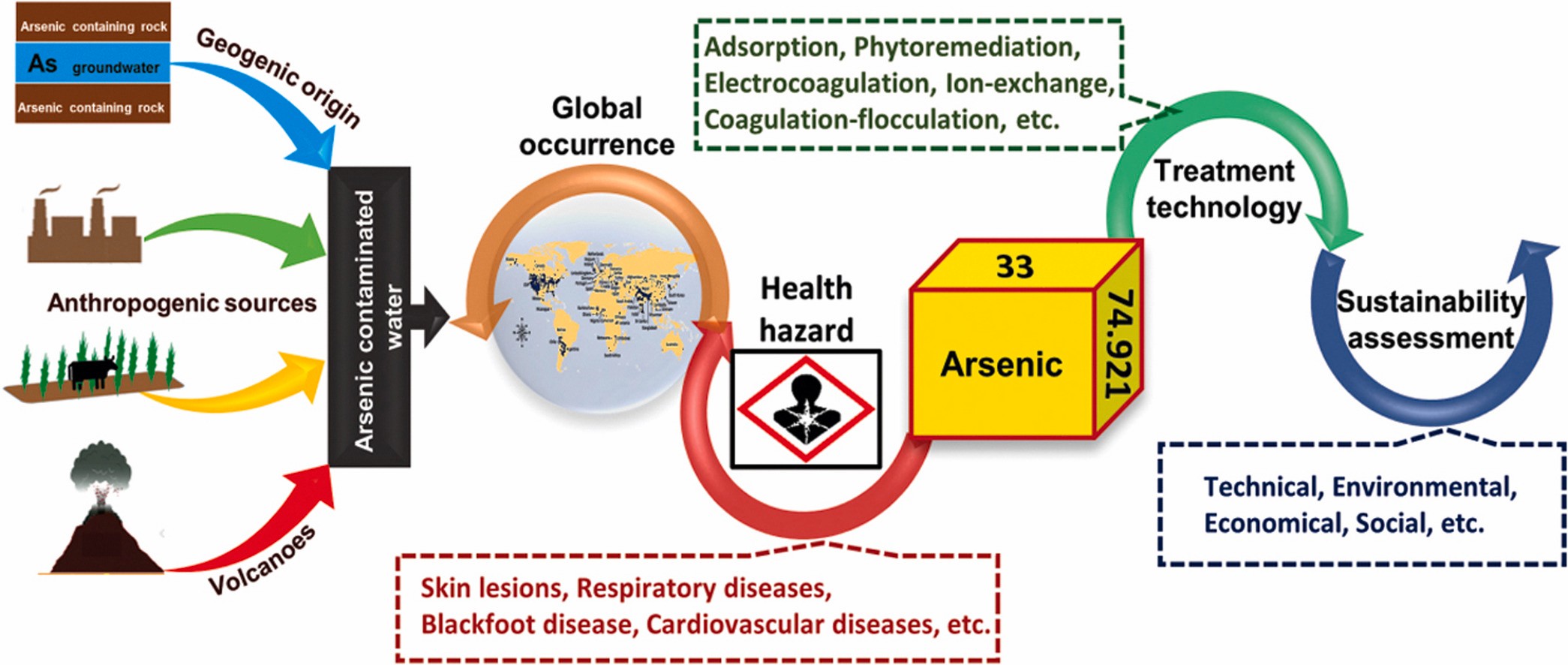
One type of drinking and industrial water pollution is arsenic contamination, which frequently results from naturally occurring high quantities of arsenic in deeper and industrial groundwater. Arsenic in drinking water has a 0.01 parts per million (ppm) limit set by the EPA. When arsenic levels in drinking water exceed 35 ppb, children are immediately at risk (0.035 ppm).
When arsenic levels in drinking water exceed 130 ppb, adults are at risk (0.130 ppm). The current WHO standard for arsenic in drinking water is 10 g/L, or 0.01 ppm, although this figure is only a guideline because it is difficult to remove arsenic from drinking water.
A 2021 study discovered that arsenic water poisoning likely affects more than 137 million people in more than seven countries.
 The issue became a severe health concern after widespread water contamination in several nations. Arsenic acid or its derivatives are frequently found in water contaminated with arsenic.
The issue became a severe health concern after widespread water contamination in several nations. Arsenic acid or its derivatives are frequently found in water contaminated with arsenic.
 These compounds are extracted from the underlying rocks that surrounds the aquifer.
These compounds are extracted from the underlying rocks that surrounds the aquifer.
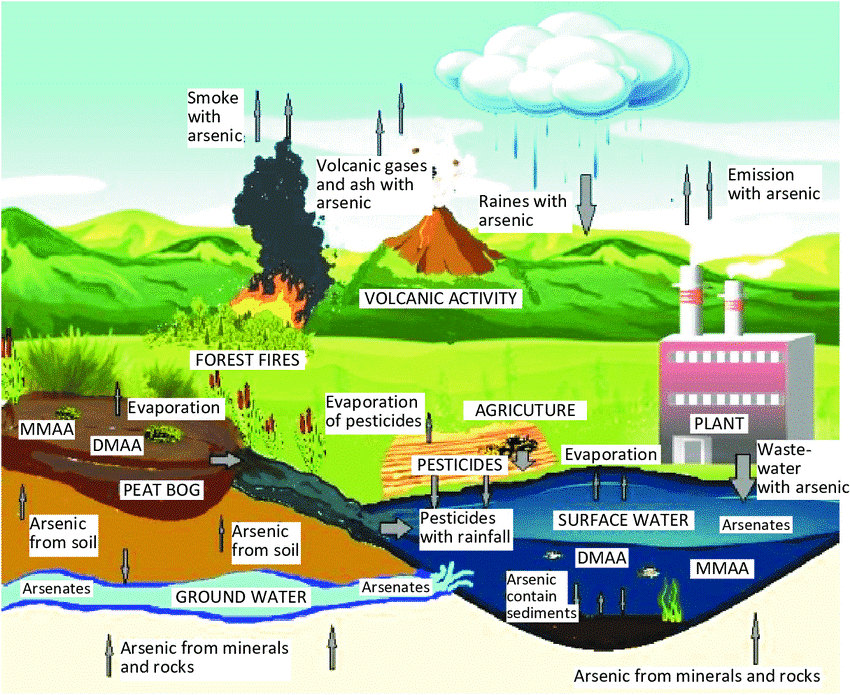 Both manmade and natural causes have been put forward for the arsenic contamination, probably in many countries, mostly man made are
Both manmade and natural causes have been put forward for the arsenic contamination, probably in many countries, mostly man made are
- Used insecticides and pesticides
- Waste disposal
- Use of arsenic treated wooden pulse
Groundwater levels were gradually dropped because of the excessive lifting of groundwater for irrigation, which opened a space for the influx of oxygen.
Arsenic content in the rocks below fluctuates because of oxygen. Inorganic arsenic is consequently discharged into the water. Since groundwater is used for domestic purposes in most rural regions and arsenic is frequently found there, arsenic contamination has become a significant public health concern.
 Arsenic builds up in the blood, which causes the skin to become harder. Arsenious deep invariably manifests as a deadly disease that progresses over a five-to-fifteen-year period. Additionally, it can cause cancer of the body’s organs, including the liver and kidneys.
Arsenic builds up in the blood, which causes the skin to become harder. Arsenious deep invariably manifests as a deadly disease that progresses over a five-to-fifteen-year period. Additionally, it can cause cancer of the body’s organs, including the liver and kidneys.
 What in the World is Arsenic
What in the World is Arsenic
It’s an element on the periodic table. It’s probably found in the earth, water, or air. Humans also add arsenic to the environment through farming and mining. People use arsenic in wood preservatives and pesticides. Arsenic exists in different forms. organic and inorganic compounds. They’re kind of like relationships. Some are more toxic than others.
 There was a 19th century Arctic explorer named Charles Francis Hall. He was perfectly healthy until he drank a cup of coffee one day and died. People thought he died from a disease, so no one suspected foul play until almost 100 years later, when a biographer looked into it. The guy discovered that Hall died from a lot of arsenic in his body, but the arsenic was only present in the last two weeks of his life.
There was a 19th century Arctic explorer named Charles Francis Hall. He was perfectly healthy until he drank a cup of coffee one day and died. People thought he died from a disease, so no one suspected foul play until almost 100 years later, when a biographer looked into it. The guy discovered that Hall died from a lot of arsenic in his body, but the arsenic was only present in the last two weeks of his life.
Arsenic can be fatal, but can also cause other health problems like
- Cancer
- Skin colour changes
- Small warts.
 Arsenic is in soil and water, some of it can make its way to drinking water and food since rice, apples and other fruits, vegetables and grains are grown in the soil.
Arsenic is in soil and water, some of it can make its way to drinking water and food since rice, apples and other fruits, vegetables and grains are grown in the soil.
There’s a teeny bit of arsenic in a bunch of food. Some plants though, like rice, take up arsenic from the soil more readily than others. For example, aspirin is helpful for minor pain relief, and is safe in small amounts, but toxic in large ones. Not just that we’re barely exposed to arsenic.
A teeny bit is in some food, but it’s not like we’re mining and around high levels of it. The EPA allows 10 ppb of arsenic in drinking water. One ppb is a sugar cube in an Olympic sized swimming pool. If more arsenic than 10 ppb is found in water, special water treatment methods are used to take it out.
 All about arsenic
All about arsenic
When we talk about arsenic being a poison, we’re talking about the compounds of arsenic like arsenic trioxide or arsenic sulphide.
 These are naturally occurring minerals known to the ancients. So, poisoning with arsenic goes back a long time. But most people, of course, are familiar with the 1944 movie Arsenic and Old Lace, or they’ve read Agatha Christie, who use arsenic as a poison, or perhaps heard stories about Napoleon who was poisoned. So, the story goes by the British, or if you don’t like that story, arsenic used in the wallpaper where he was imprisoned, leached out into the air and that poisoned him.
These are naturally occurring minerals known to the ancients. So, poisoning with arsenic goes back a long time. But most people, of course, are familiar with the 1944 movie Arsenic and Old Lace, or they’ve read Agatha Christie, who use arsenic as a poison, or perhaps heard stories about Napoleon who was poisoned. So, the story goes by the British, or if you don’t like that story, arsenic used in the wallpaper where he was imprisoned, leached out into the air and that poisoned him.
 In ancient Korea, though, the kings had a different idea. They knew something about chemistry, they knew something about silver and tarnishing.
In ancient Korea, though, the kings had a different idea. They knew something about chemistry, they knew something about silver and tarnishing.
Arsenic sulphide will react with silver to tarnish the silver and the ancient Korean kings are aware of this. So, what did they do to detect arsenic in the food if there was any, they would resort to silver chopsticks. The idea was that if there was arsenic sulphide in the food, the silver would tarnish not likely to have worked because you need quite a bit of arsenic to tarnish the silver and furthermore, it doesn’t happen right away. Also, there are other foods that contain sulphites like garlic, so if they were eating garlic, that also would have tarnished silver.
 Five Things to Know About Arsenic in Drinking Water and standard
Five Things to Know About Arsenic in Drinking Water and standard
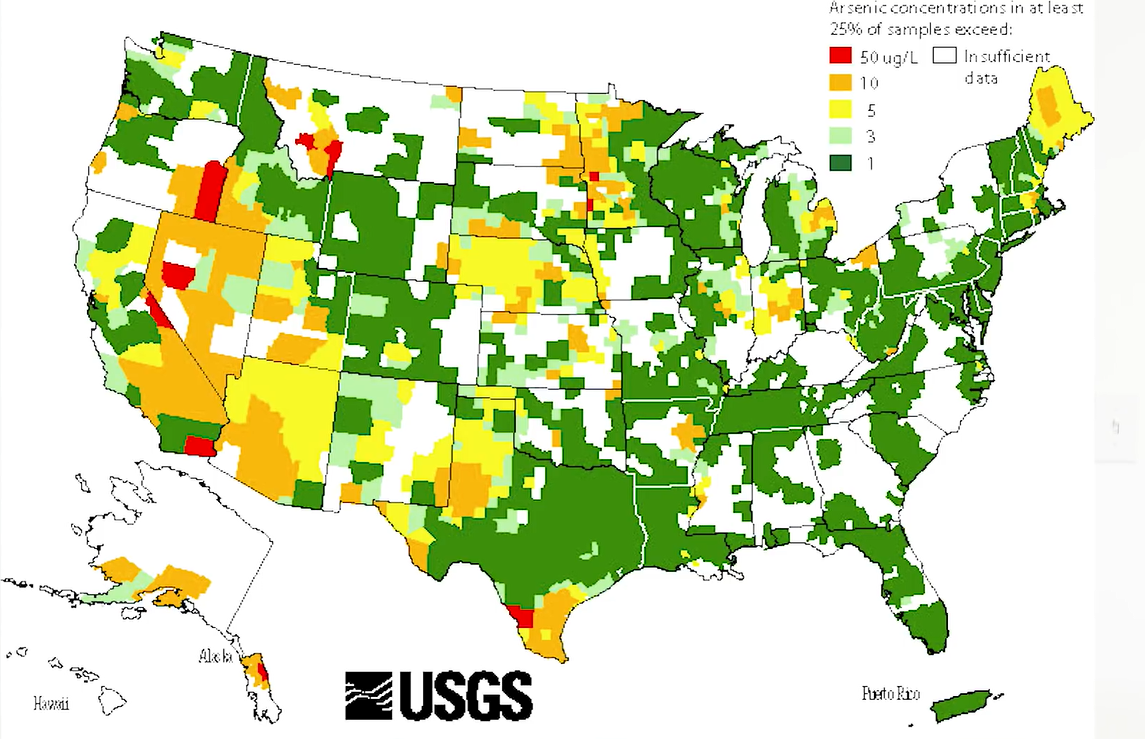
Lead which leeches from plumbing or chromium six which comes from industrial pollution, arsenic enters your drinking water from rocks that contain arsenic. This means that your water could be contaminated based only on the natural geology. It’s not fair, but it’s how it is. This map from USGS shows areas where arsenic levels are high.
 There are two main types found in drinking water,
There are two main types found in drinking water,
- Arsenic three and
- Arsenic five.
They are both toxic, but it’s important to understand that there are two forms when we start talking about water filtration a bit later.
Arsenic regulations are pretty loose considering there’s no level known to be safe. EPA is current drinking water limit is 10 parts per billion. However, municipalities with lower levels are required to include the following disclosure language in their annual drinking water report.
To test your home’s water for arsenic you need to send a water sample to an accredited laboratory. TDS metres do not tell you anything about arsenic levels in your water and test kits from big box retailers are not sensitive enough to get meaningful results.
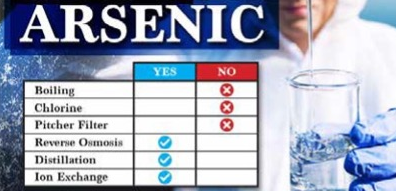
 If you want to remove arsenic from your home’s water, you need to use a water filter that is designed to remove the type of arsenic found in your water. Boiling your water does not remove arsenic nor do common pitchers and fridge filters. While arsenic five is easily removed by several technologies, including simple ion exchange media and reverse osmosis. Arsenic three is more difficult to remove and requires more advanced filtration.
If you want to remove arsenic from your home’s water, you need to use a water filter that is designed to remove the type of arsenic found in your water. Boiling your water does not remove arsenic nor do common pitchers and fridge filters. While arsenic five is easily removed by several technologies, including simple ion exchange media and reverse osmosis. Arsenic three is more difficult to remove and requires more advanced filtration.
 Removal of arsenic from industrial wastewater
Removal of arsenic from industrial wastewater
The poisonous heavy metal element arsenic is frequently present in the wastewater released from the chemical and metallurgical sectors. The environment would suffer significant harm if wastewater were dumped into the environment untreated.
Arsenic’s development has coincided with the growth of the metallurgical and chemical sectors as well as the mining of low-grade ores. The main sources of arsenic in sewage include ore mining, burning fossil fuels, smelting non-ferrous metals, making pharmaceuticals with arsenic, volcanic eruption, arsenic-solidified wastes, and pesticide use. All arsenic compounds, including arsenic, are extremely poisonous protoplasts. Arsenic pollution has a negative impact on a lot of nations. Uncontrolled discharge of non-wastewater containing arsenic will seriously endanger human health and the environment.
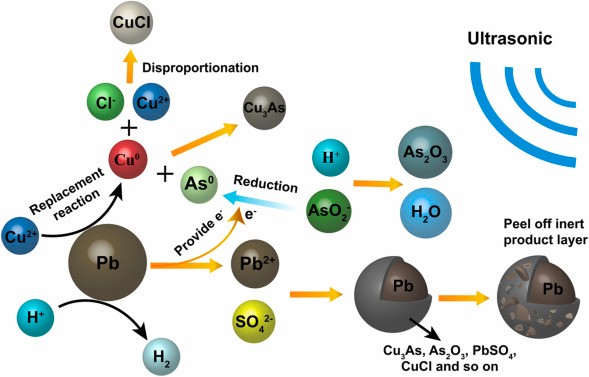 Arsenic can impair cellular metabolism and result in cell death. Keratinization of the skin and even cancer can result from chronic arsenic poisoning. To maintain the water environment and guarantee the safety of drinking and industrial water, it is crucial to manage the amount of arsenic present. Concern across the globe has shifted to the safe treatment of industrial effluent that contains arsenic.
Arsenic can impair cellular metabolism and result in cell death. Keratinization of the skin and even cancer can result from chronic arsenic poisoning. To maintain the water environment and guarantee the safety of drinking and industrial water, it is crucial to manage the amount of arsenic present. Concern across the globe has shifted to the safe treatment of industrial effluent that contains arsenic.
Wastewater treatment for high levels of arsenic
The primary source of the high arsenic wastewater is the acidic waste created when copper and other non-ferrous metals are smelted. Acidic heavy metal effluent, residential wastewater, and recirculating cooling water are the main sources of waste acid. Sewage is abundant, and its makeup is intricate. Additionally, wastewater with high arsenic levels contains hazardous metal ions such as bismuth, cadmium, lead, zinc, copper, and arsenic.
So, it must be given additional treatment and disposed in accordance with standards. Chemical precipitation, which produces solid arsenic compounds, is the primary approach for reducing arsenic content in wastewater for large concentrations of arsenic-containing wastewater. It is crucial that we eliminate arsenic from waste acid from the smelting of non-ferrous metals, achieve discharge of waste acid up to the standard, and implement water recycling to protect the environment.
- Lime and iron salt elimination of arsenic
- Sulfurization for the elimination of adenine.
 Wastewater treatment with low levels of arsenic
Wastewater treatment with low levels of arsenic
- Adsorption-based elimination of arsenic
- Ion exchange for the elimination of arsenic
- Membrane separation for the removal of arsenic.
 Arsenic free drinking water
Arsenic free drinking water
The world’s streams, lakes, oceans, and wells are all made of water, a translucent liquid. No known life form exists without it that covers 71% of Mother Earth. However, for many of our fellow citizens, safe water is a dream, the water resources are contaminated naturally with deadly arsenic and nonmetal with atomic number 33. Continual ingestion of poisonous arsenic can lead to.
- Skin cancer
- Ailments of lungs,
- Bladder and kidney and eventual death.
 Nano technology-based filter which has a flow rate of 50 to 70 litres per hour. That was ideal for placing in places where it could be used as a standalone unit. It could serve more than 100 families.
Nano technology-based filter which has a flow rate of 50 to 70 litres per hour. That was ideal for placing in places where it could be used as a standalone unit. It could serve more than 100 families.
 So, we think this was a very ideal solution. In such situations where you have remote areas where you have hardly any power supply.
So, we think this was a very ideal solution. In such situations where you have remote areas where you have hardly any power supply.
Last year we have installed around 30 units of nanotechnology based arsenic filtration units have found it to be very effective, cost effective, and more importantly, speedy technology for tackling one of the dangerous ministers of our country.
The technology has also been implemented in high volume discharge applications and has found acceptance among the local populace. Approximately around 50 lakhs of population are affected by arsenic contamination and the water.
This technology is based on nanotechnology. And so far, more than 500 community locations, this technology is being used. And the feedback from the people is really very good and encouraging. This particular technology removes not only arsenic, but also iron. The benefits have started showing lesser and lesser number of people are reporting sick due to water related causes. These water purifiers are extremely simple to maintain. The technology is now delivering clean water to 200,000 people.
 Arsenic Removal from drinking and Industrial water
Arsenic Removal from drinking and Industrial water
Arsenic is a trace component of many rocks and minerals. As you can see below, it is ubiquitous throughout the world, dangerously high levels are found in the drinking and industrial water in more than 25 states in the US, potentially exposing 2.1 million people to harmful levels of arsenic.
 More than 100 million people were poisoned in Bangladesh, where arsenic poisoning was at its worst. A total of 150 million individuals are exposed to arsenic through drinking water worldwide. Various businesses and animal feed both use arsenic. It serves as a pesticide and a wood preservative. It is in poultry and swine feed to enhance growth, improve feed efficiency, prevent parasitic diseases, and provide the farmer with bigger returns on their livestock.
More than 100 million people were poisoned in Bangladesh, where arsenic poisoning was at its worst. A total of 150 million individuals are exposed to arsenic through drinking water worldwide. Various businesses and animal feed both use arsenic. It serves as a pesticide and a wood preservative. It is in poultry and swine feed to enhance growth, improve feed efficiency, prevent parasitic diseases, and provide the farmer with bigger returns on their livestock.
Below map shows the arsenic concentrations in groundwater across the US. In Michigan, the highest levels of arsenic are observed in the thumb area. The maximum contaminant level for drinking waters in the United States is 10 micrograms per litre.
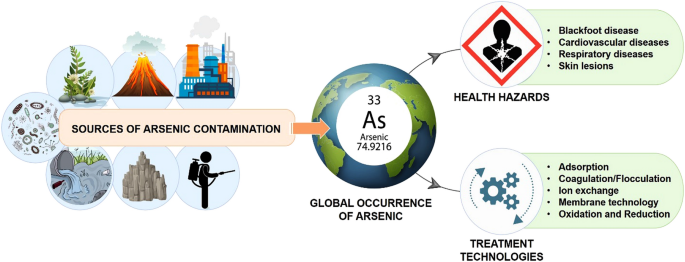 Arsenic exists in the environment in three oxidation states,
Arsenic exists in the environment in three oxidation states,
- Arsenic zero
- Arsenic three plus
- Arsenic five plus
Arsenic zero only exists in highly reducing conditions. For example, in sediments, arsenite exists in five forms as
- Tetra hydrogen arsenite or
- Arsenous acid
- Dihydrogen arsenite
- Hydrogen arsenide, and
- Arsenite
 Most common environmental conditions, where the pH is between 6 to 8, Arsenic three occurs predominantly in the Arsenous acid form and to some extent as the dihydrogen arsenite
Most common environmental conditions, where the pH is between 6 to 8, Arsenic three occurs predominantly in the Arsenous acid form and to some extent as the dihydrogen arsenite
Arsenate exists in four forms and aqueous solutions; this is based on pH.
It occurs as
- Arsenic acid
- Dihydrogen arsenite,
- Hydrogen arsenite
- Arsenite
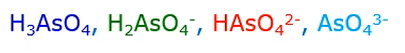 Under most environmental conditions the predominant arsenic five forms are
Under most environmental conditions the predominant arsenic five forms are
- Dihydrogen arsenite and
- Hydrogen arsenite.
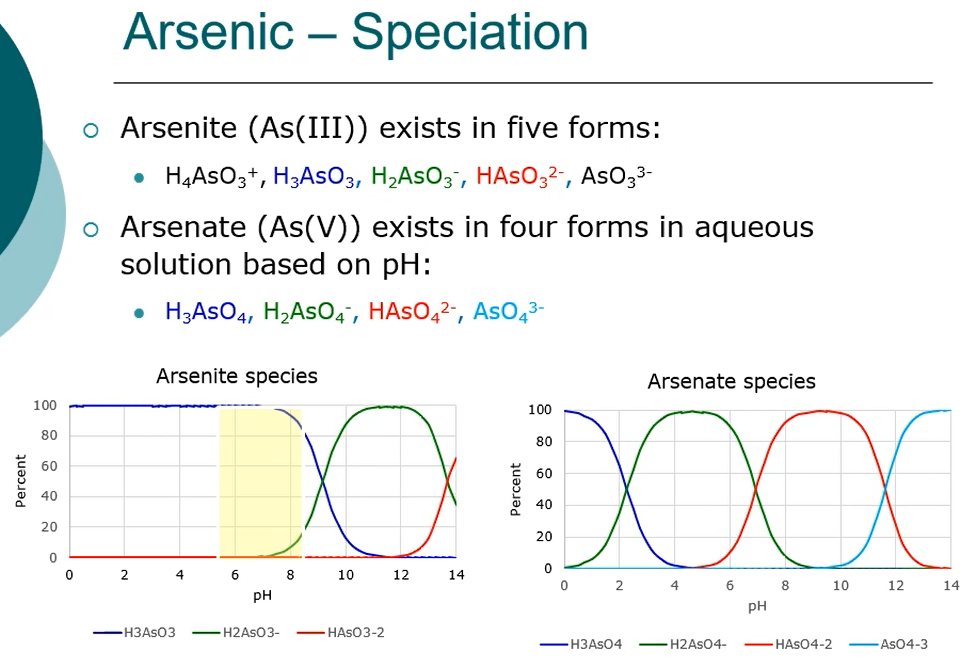 Shown in this figure below the Eh- pH diagram for arsenic at 25 degrees C in one atmosphere at most natural pH waters and in toxic environments,
Shown in this figure below the Eh- pH diagram for arsenic at 25 degrees C in one atmosphere at most natural pH waters and in toxic environments,
Arsenate predominates in the ionic forms of dihydrogen arsenite and hydrogen arsenite
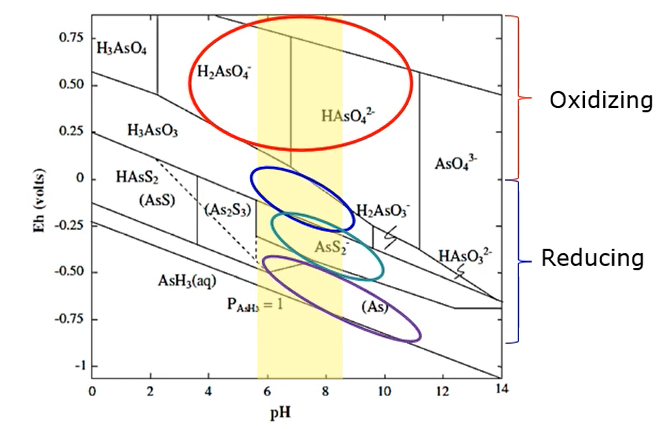 In less oxidising environments at Circle neutral pH are arsenic acid predominates. And in reducing conditions, where a sulphide is present arsenic is present as arsenite sulphide.
In less oxidising environments at Circle neutral pH are arsenic acid predominates. And in reducing conditions, where a sulphide is present arsenic is present as arsenite sulphide.
Arsenic treatment Mechanisms
There are numerous ways by which arsenic can be treated.
They can be grouped into five categories,
- Oxidation,
- Coagulation, flocculation,
- Adsorption,
- Ion exchange and
- Membrane technologies.
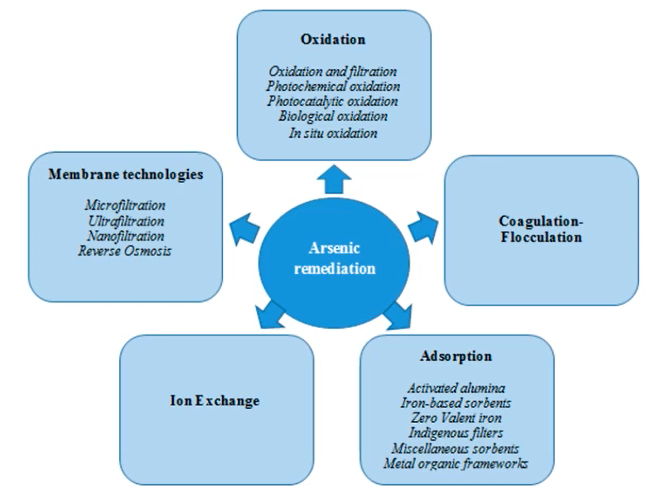 The first two are not truly removal mechanisms, as for example, oxidation, simply convert the arsenic form from the reduced states to a more oxidised state. coagulation and flocculation are not a removal mechanism in less the water is treated subsequently by either sedimentation or by filtration.
The first two are not truly removal mechanisms, as for example, oxidation, simply convert the arsenic form from the reduced states to a more oxidised state. coagulation and flocculation are not a removal mechanism in less the water is treated subsequently by either sedimentation or by filtration.
However, both of these processes result in the presence of arsenic in the residual. In this portion, we’ll focus on coagulation flocculation followed by filtration.
Arsenic removal: Coagulation
In order for arsenic to be effectively removed by coagulation with iron salts, that is either ferrous chloride, ferric chloride or ferric sulphate, arsenic three must first be oxidised. The most common Oxidants are chlorine and ozone. At the same time any reduced iron and manganese can also be oxidised.
 As iron five attaches to the iron hydroxides through adsorption and or Coprecipitation it can be removed in this way the particles or precipitates are then filtered from the water.
As iron five attaches to the iron hydroxides through adsorption and or Coprecipitation it can be removed in this way the particles or precipitates are then filtered from the water.
As shown in this graph ferric sulphate is a much more effective coagulant then is alum. The removal efficiency of ferric sulphate drops off considerably at pH values greater than 8.5 and add pH is greater than approximately seven for alum. As a result, ferric sulphate is a much better coagulant over a wider pH range, then is alum.
 Arsenic removal by Lime Soda Ash Softening
Arsenic removal by Lime Soda Ash Softening
Arsenic can also be removed using lime soda ash softening with excess lime for manganese removal 60 to 90% removal of arsenic can be achieved.
 However, with single stage softening plants that only remove calcium up to 40% removal can be achieved. It should be noted that arsenic five is much more readily removed than is arsenite or arsenic three. Thus, oxidation before softening is recommended. The major removal mechanism is by adsorption to the calcium carbonate or magnesium hydroxide precipitate.
However, with single stage softening plants that only remove calcium up to 40% removal can be achieved. It should be noted that arsenic five is much more readily removed than is arsenite or arsenic three. Thus, oxidation before softening is recommended. The major removal mechanism is by adsorption to the calcium carbonate or magnesium hydroxide precipitate.
This was the overview of methods to remove arsenic from drinking and Industrial waters.
Ultra-low-cost filter for arsenic removal from drinking and industrial water
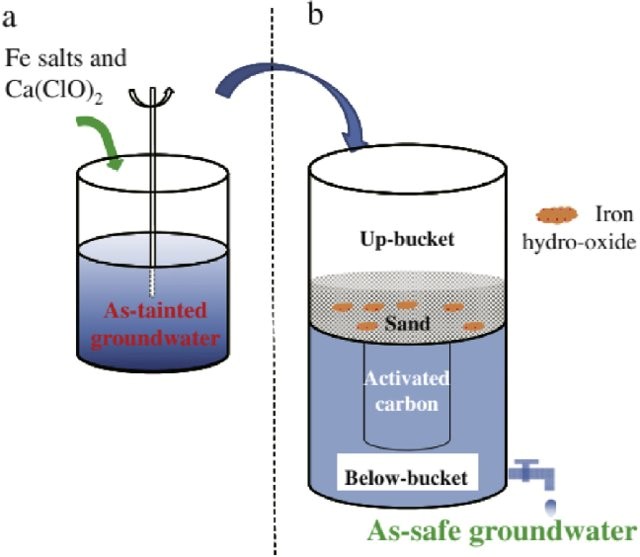
Waterman Australia has developed a completely indigenous ultra-low-cost adsorbent filter capable of absorbing arsenic. This easy-to-handle filter is made from Red late right soil and has the capacity to absorb arsenic to the extent of 32 mg per gramme of material.
 Project is on arsenic removal from drinking and industrial water or from the groundwater. And the major point of the project is that it is ultra-low-cost and the technology that we’re using it is absorption using a naturally occurring material.
Project is on arsenic removal from drinking and industrial water or from the groundwater. And the major point of the project is that it is ultra-low-cost and the technology that we’re using it is absorption using a naturally occurring material.
Arsenic filters benefits
Compared to other search available filters, this product is appropriate for the socio-economic conditions of many countries.
- The life of this filter is extremely long, about five years,
- No regeneration of the absorbent is required during its lifetime.
- There is no requirement of electricity for the household filter,
- The capacity of domestic filter is in the range of 80 to 100 litres per day,
- Cost of the treated water is less than other available filter per litre
 Arsenic in a single filter you can remove arsenic, iron or microorganism at a time. Third point is that we can use this filter in a remote village or that there is no electricity is available. So, this filter can run without any electricity. Fourth point is that it has a very long life about five to seven years and the material that is used for the arsenic removal after its life is over. This can be you know utilised it satisfies a TCLP protocol.
Arsenic in a single filter you can remove arsenic, iron or microorganism at a time. Third point is that we can use this filter in a remote village or that there is no electricity is available. So, this filter can run without any electricity. Fourth point is that it has a very long life about five to seven years and the material that is used for the arsenic removal after its life is over. This can be you know utilised it satisfies a TCLP protocol.
This indigenous filter for arsenic removal is low cost and easy to handle. With this technology the people of world can avail arsenic free water.
Arsenic contamination of ground water
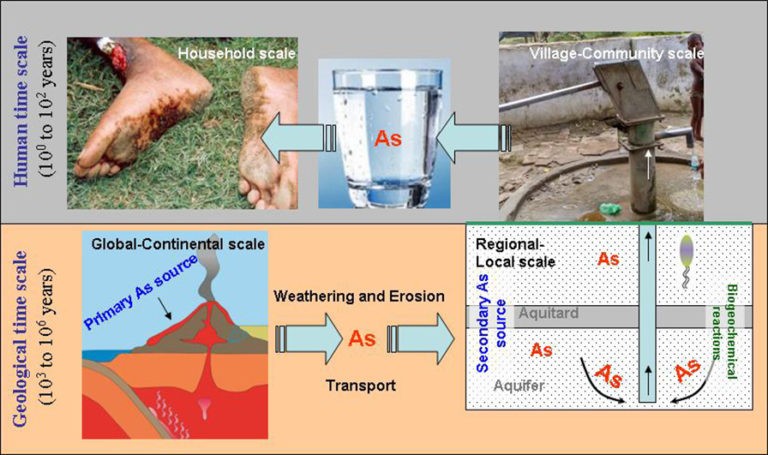
Groundwater contamination from arsenic is a geogenic contaminant, meaning it results from a normal geologic process. 2.34 billion people reside in rural areas where there are significant levels of arsenic in the groundwater, and 78% of the people in the state live in rural areas and depend on groundwater for drinking, cooking, and irrigation.
 United States and government production agency set an arsenic maximum limit for public water usage. That is 0.010 milligrams per litre, which is equivalent to 0.010 parts per million (ppm) occurrences of elevated groundwater arsenic levels recorded across the nation.
United States and government production agency set an arsenic maximum limit for public water usage. That is 0.010 milligrams per litre, which is equivalent to 0.010 parts per million (ppm) occurrences of elevated groundwater arsenic levels recorded across the nation.
If your well water turns out to be contaminated, there are a few options to regain potable water in your household.
- The first option is to join a public water supply system.
- The second option is to instal an arsenic filter for your well.
- The last option is to drill or switch to a new well that is more likely to produce arsenic free groundwater.
In less developed countries the last option is often preferred due to the absence of a public water supply system or effective water monitoring and treatment equipment.
 Waterman Australia have built many study sites and arsenic affected regions. The research effort has also motivated programmes that are in place to help affected populations regain clean drinking water.
Waterman Australia have built many study sites and arsenic affected regions. The research effort has also motivated programmes that are in place to help affected populations regain clean drinking water.
Household measures to reduce arsenic exposure
The bore well in your household and Village is an important source of water for many things like
- Drinking
- Watering crops
- Bathing
- Washing clothes
- Food preparation.
 However, did you know that the groundwater from these borewells in certain parts of the countries is contaminated with a naturally occurring poison that you cannot see or taste. This poison is called arsenic and if you consume it over a long period, it can make you and your family very sick and even cause deadly disease such as cancer.
However, did you know that the groundwater from these borewells in certain parts of the countries is contaminated with a naturally occurring poison that you cannot see or taste. This poison is called arsenic and if you consume it over a long period, it can make you and your family very sick and even cause deadly disease such as cancer.
This poisoned groundwater finds its way into food crops such as rice, wheat, and vegetables like potato. To protect your family’s health. You need to make sure you drink safe water, but also just as importantly, prepare and cook food using safe water. When you cook your rice First, wash the rice grains thoroughly in safe water, then parboil them and drain the water which has drawn the arsenic.
 Finally, boil it in excess amount of clean safe water until rice is cooked. You should never boil contaminated water trying to make it safe. It makes it worse, and arsenic is not removed.
Finally, boil it in excess amount of clean safe water until rice is cooked. You should never boil contaminated water trying to make it safe. It makes it worse, and arsenic is not removed.
 Is there arsenic in your drinking water
Is there arsenic in your drinking water
If you’re drinking water from a private well. The only way to know for sure is to have your water tested. Arsenic is a natural element so often found at low levels throughout our environments and across the world. Arsenic is common in rocks and soil sometimes at high levels. As water passes from the surface down through the layers of bedrock, it may pull arsenic into groundwater aquifers.
 Aquifers supply drinking water for private wells and many city water systems. Cities are required to regularly test water for many contaminants, including arsenic. Put private well owners are not required to test their drinking water. Why is this a problem? Because arsenic is highly toxic. Throughout history Arsenic has been referred to as the king of poisons because when it is dissolved in liquid like drinking water, it has no colour, taste, or smell.
Aquifers supply drinking water for private wells and many city water systems. Cities are required to regularly test water for many contaminants, including arsenic. Put private well owners are not required to test their drinking water. Why is this a problem? Because arsenic is highly toxic. Throughout history Arsenic has been referred to as the king of poisons because when it is dissolved in liquid like drinking water, it has no colour, taste, or smell.
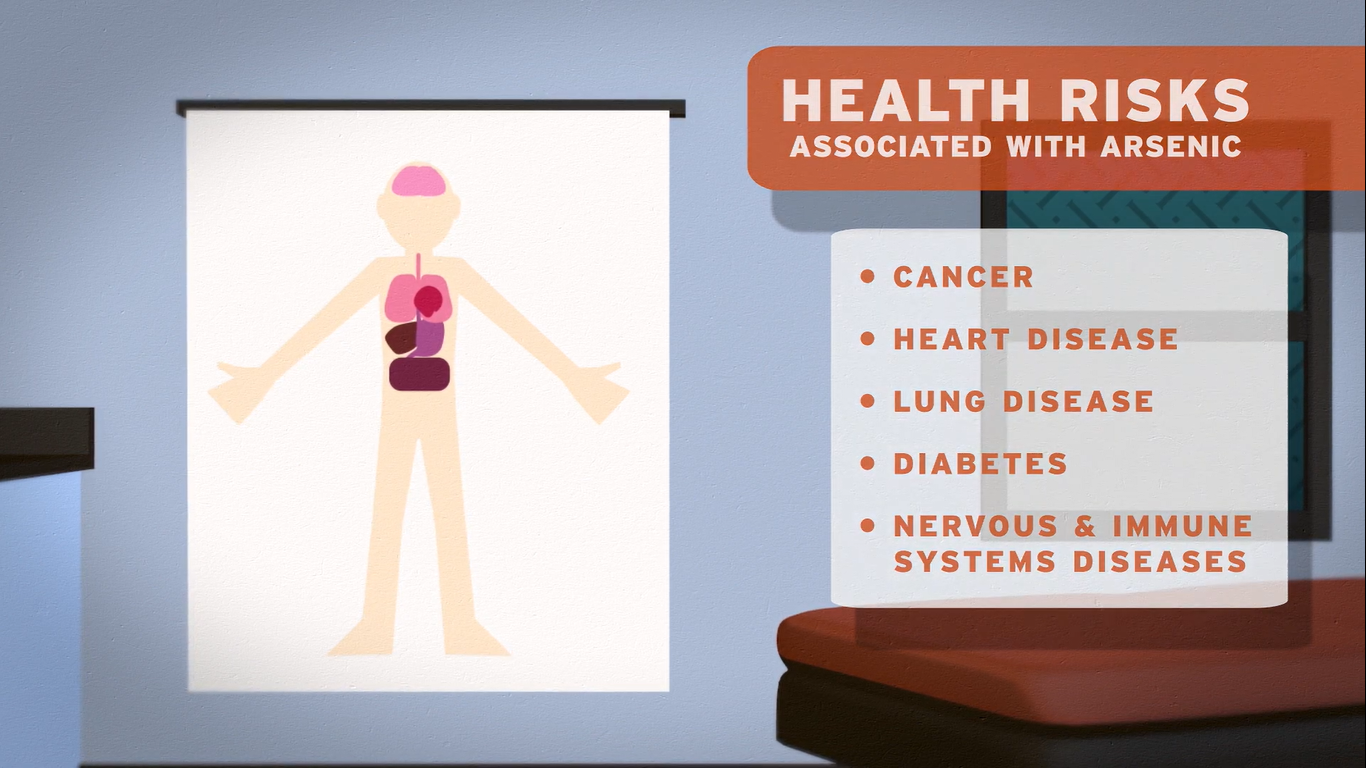
 Drinking water with a high level of arsenic can have immediate and severe health risks. Even if arsenic is present at low levels, drinking it for a long period of time poses health risks. You could go many years without symptoms, however, prolonged exposure to arsenic can result in cancer, heart disease, lung disease, diabetes and diseases of the nervous and immune systems.
Drinking water with a high level of arsenic can have immediate and severe health risks. Even if arsenic is present at low levels, drinking it for a long period of time poses health risks. You could go many years without symptoms, however, prolonged exposure to arsenic can result in cancer, heart disease, lung disease, diabetes and diseases of the nervous and immune systems.
 If you know that arsenic is in your drinking water, you can take steps to remove. This will help ensure that your water is safe from this silent poison.
If you know that arsenic is in your drinking water, you can take steps to remove. This will help ensure that your water is safe from this silent poison.
Removing Arsenic from Drinking Water
For millions of people in the US and tens of millions worldwide, access to clean drinking water is a problem on a national and international level. The presence of arsenic contamination poses a concern to health. Arsenic drinking water limits have just been lowered by the EPA to 10 parts per billion (ppb), putting the health of 4000 municipalities and approximately 14 million individual households at danger. However, modern technology now provides a remedy for resuming the delivery of safe water to families.
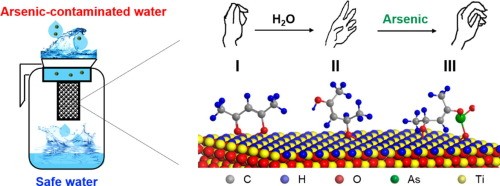 The best material currently on the market is seven times less effective than the revolutionary material that nanotechnology researchers at Waterman Australia have created, called nano composite arsenic sorbent. This absorbent contains extremely high concentrations of arsenic absorbing nanoparticle metal oxides embedded in a strong composite polymer matrix, as shown in this series of micrographs.
The best material currently on the market is seven times less effective than the revolutionary material that nanotechnology researchers at Waterman Australia have created, called nano composite arsenic sorbent. This absorbent contains extremely high concentrations of arsenic absorbing nanoparticle metal oxides embedded in a strong composite polymer matrix, as shown in this series of micrographs.
The large surface area of this material and the reaction kinetics strength that distinguish it from all other arsenic removal techniques allow for the practically total removal of arsenic from running water. Sorbent particles have a surface area similar to that of carbon nanotube materials. Because sorbent is so effective, one gramme of it has 40% more surface area than the typical American home’s square footage. These nanoparticles can efficiently remove arsenic from 350 to 400,000 gallons of water in one gallon, as opposed to the next-best material’s 50,000 gallons.
 In addition, it is stronger and has a lifespan 100 times longer than that of the toughest substance now in use. In addition to being very efficient, sorbent is also quite inexpensive, treating 1000 gallons of water with it only costs approximately 10 cents. the price of available treatment methods.
In addition, it is stronger and has a lifespan 100 times longer than that of the toughest substance now in use. In addition to being very efficient, sorbent is also quite inexpensive, treating 1000 gallons of water with it only costs approximately 10 cents. the price of available treatment methods.
Arsenic contamination is a worldwide issue that results in disease, needless suffering, and skin sores. A low cost, highly efficient arsenic removal technique is required by 10s of thousands of towns and 10s of millions of individuals. Customers here and throughout the world can access healthier water thanks to a cost-effective arsenic treatment solution from Waterman Australia’s nanomaterial.
Ion Exchange Arsenic treatment technology
The purpose of this blog is to provide small water systems with an introduction to the use of anion exchange as an arsenic removal technology. The greatest technology currently available for eliminating arsenic from drinking water with low sulphate concentrations is ion exchange.
 Ion exchange as a treatment process that uses synthetic resin that exchange an ion for an unwanted ion in water. Many people are familiar with this process because it is often used in homes to remove hardness from water. A packed bed of ion exchange resin bead is employed to carry out the exchange reaction, and raw water is repeatedly circulated through the resin bed until the resin is nearly depleted.
Ion exchange as a treatment process that uses synthetic resin that exchange an ion for an unwanted ion in water. Many people are familiar with this process because it is often used in homes to remove hardness from water. A packed bed of ion exchange resin bead is employed to carry out the exchange reaction, and raw water is repeatedly circulated through the resin bed until the resin is nearly depleted.
 If the resin is operated beyond exhaustion, breakthrough of the unwanted ion or ions will occur. Arsenic five ions that are negatively charged are exchanged for the chloride ions of virgin resin while removing arsenic using an ion exchange resin.
If the resin is operated beyond exhaustion, breakthrough of the unwanted ion or ions will occur. Arsenic five ions that are negatively charged are exchanged for the chloride ions of virgin resin while removing arsenic using an ion exchange resin.
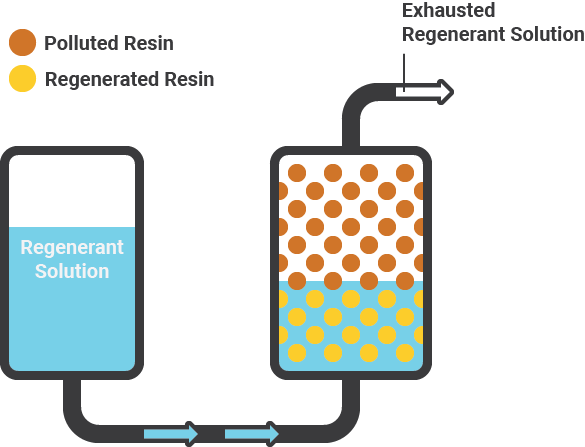
Regeneration of resin
The exchange process has reversed by flushing the resin with a concentrated sodium chloride brine solution. This causes the resin to release the arsenic five and other and ions by replacing them with chloride from the brine and prepares the resin for reuse. It is important to remember that only the oxidised form of arsenic.
 Arsenic five can be removed using an ion exchange process. The process is not effective for the removal of arsenic three. Equally important is the fact that most resins preferentially remove sulphate over arsenic and nitrate. The resin loads from the top downward with the flow of raw water. As the resin bed becomes exhausted sulphate forces the arsenic and nitrate deeper into the bed.
Arsenic five can be removed using an ion exchange process. The process is not effective for the removal of arsenic three. Equally important is the fact that most resins preferentially remove sulphate over arsenic and nitrate. The resin loads from the top downward with the flow of raw water. As the resin bed becomes exhausted sulphate forces the arsenic and nitrate deeper into the bed.
 The treatment process must be stopped for regeneration of the resin prior to full exhaustion, or the sulphate can force nitrate and arsenic to be discharged into the finished water at concentrations much higher than in the raw water. This phenomenon known as chromatographic peaking can cause health risks particularly so for nitrate which is an acute contaminant because of the water contains orders of magnitude more sulphate and nitrate rate than arsenic. The frequency of regeneration is dictated by the sulphate and nitrate concentrations.
The treatment process must be stopped for regeneration of the resin prior to full exhaustion, or the sulphate can force nitrate and arsenic to be discharged into the finished water at concentrations much higher than in the raw water. This phenomenon known as chromatographic peaking can cause health risks particularly so for nitrate which is an acute contaminant because of the water contains orders of magnitude more sulphate and nitrate rate than arsenic. The frequency of regeneration is dictated by the sulphate and nitrate concentrations.
The raw water is produced by a single well capable of up to 200 gallons per minute. The water contains about 35 micrograms per litre arsenic 51 milligrams per litre of sulphate from 12 to 15 milligrams per litre nitrate and has a pH of about 7.3.
Most of our arsenic ranges from 20 parts per billion to about 38 parts per billion. And some of our whales are low in nitrate, but over 50% of the well are high in what I call borderline nitrates they’ll be anywhere from six milligrams per litre to nine milligrams per litre.
The treatment process consists of
- A battery of 25-micron bag filters to remove sand or other sediment
- Skid mounted ion exchange vessels, and brin production and storage facilities for regeneration of the resin.
 The groundwater contains primarily the oxidised form of arsenic, arsenic five, therefore, pre-treatment with an oxidant is not necessary, although some states might require chlorination of the treated water to ensure microbial quality. Waterman Australia selected the ion exchange because in addition to arsenic five, it also removes nitrate which is present at levels above the maximum contaminant level,
The groundwater contains primarily the oxidised form of arsenic, arsenic five, therefore, pre-treatment with an oxidant is not necessary, although some states might require chlorination of the treated water to ensure microbial quality. Waterman Australia selected the ion exchange because in addition to arsenic five, it also removes nitrate which is present at levels above the maximum contaminant level,
Probably will most likely have to use ion exchange because of the borderline nitrates. We don’t want to put a capital expense into removing arsenic and then find out six months or one year later that that borderline nitrate is elevated and exceeded the 10 milligrams per litre and therefore have to shut the water source down.
After passing through the bag filters, the raw water stream is split equally to flow from top to bottom half through each resin filled vessel. This is called a parallel configuration.
Then the arsenic and the nitrate stick to the resin come at the bottom and away they go and then then in a regeneration, it also feeds from top to bottom.
 Since the raw water concentrations of sulphate arsenic and nitrate are relatively stable, the process is predictable, and has been adjusted such that regeneration of each vessel occurs after treatment of 335,000 gallons of water.
Since the raw water concentrations of sulphate arsenic and nitrate are relatively stable, the process is predictable, and has been adjusted such that regeneration of each vessel occurs after treatment of 335,000 gallons of water.
When regeneration is called for a portion of the raw water flow for one vessel is diverted through a venturi that pulls in a concentrated brine solution to be mixed with the remainder of the water and then pass through the resin.
 Chloride ions from the brine replace the sulphate, arsenic and nitrate attached to the exhausted resin. Salt for regeneration is stored in this container that is filled to the six-foot mark with water to ensure a constant supply of brine.
Chloride ions from the brine replace the sulphate, arsenic and nitrate attached to the exhausted resin. Salt for regeneration is stored in this container that is filled to the six-foot mark with water to ensure a constant supply of brine.
 There’s a water level from the bottom of the top it’s right about oh right in here somewhere it’s about six-foot, six foot one, it’ll vary from six one to five, seven on any given day. When you go into a regeneration, this valve will kick open off the timer from the computer. it’ll fill this up to the demand need. And that’s how you get yourself saturation brin for the regeneration,
There’s a water level from the bottom of the top it’s right about oh right in here somewhere it’s about six-foot, six foot one, it’ll vary from six one to five, seven on any given day. When you go into a regeneration, this valve will kick open off the timer from the computer. it’ll fill this up to the demand need. And that’s how you get yourself saturation brin for the regeneration,
The Waterman Australia uses from 6000 to 8000 pounds salt per week at a delivered cost of about 10 cents per pound. The waste brine containing the arsenic and the rinse water is discharged to a floor drain from which it is transferred to the sanitary sewer.
Waste brine produced by the ion exchange process may contain enough arsenic to be considered hazardous. Therefore, ion exchange is often more cost effective when the brin can be discharged to a sanitary sewer.
 Ion Exchange is also used for arsenic removal in other countries. This system is a proprietary and ion exchange process provided by basin water. The treatment vessels, pumps, controls etc. are delivered to a prepared site in a shipping container that contains several vessels operated in parallel.
Ion Exchange is also used for arsenic removal in other countries. This system is a proprietary and ion exchange process provided by basin water. The treatment vessels, pumps, controls etc. are delivered to a prepared site in a shipping container that contains several vessels operated in parallel.
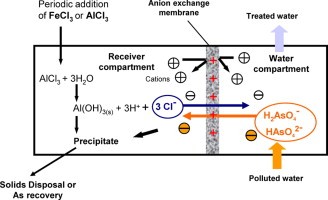 The basin water system has been used at numerous locations for nitrate removal and is now being used for arsenic removal. Water Man Australia Company has selected basins ion exchange are several sites because it was found through a bidding process to be the most cost-effective technology. Typically, all but three vessels are in production. One vessel is in standby mode, one in the process of regeneration, and one offline for regeneration.
The basin water system has been used at numerous locations for nitrate removal and is now being used for arsenic removal. Water Man Australia Company has selected basins ion exchange are several sites because it was found through a bidding process to be the most cost-effective technology. Typically, all but three vessels are in production. One vessel is in standby mode, one in the process of regeneration, and one offline for regeneration.
 One advantage of multiple vessels is that a more constant flow of finished water can be produced the two-vessel system loses 50% of its production for nearly five hours during regeneration. Since an ion exchange also removes bicarbonate alkalinity. The multiple vessels stage regeneration approach provides finished water with a more stable pH and alkalinity
One advantage of multiple vessels is that a more constant flow of finished water can be produced the two-vessel system loses 50% of its production for nearly five hours during regeneration. Since an ion exchange also removes bicarbonate alkalinity. The multiple vessels stage regeneration approach provides finished water with a more stable pH and alkalinity
This can be important for lead and copper control. This system is controlled by a computerised system that can be accessed remotely. It reuses brine three times and uses rinse water for Brian production, therefore reportedly produces a lesser quantity of waste. In some locations, the waste brine is passed through absorptive media that will remove the arsenic.
We have our absorptive media and here we circulate the waste brine solution across this media and the arsenic then attaches to the median here and now we have brine solution which is low and arsenic. The arsenic is left behind in these drums, and at that point, the waste brine is then transferred to the waste tanks outside for disposal.
That media typically passes the toxicity characteristics leaching potential test, the treat of brine is then hauled away for disposal.
Summary ion exchange method
In summary, ion exchange may be a suitable arsenic removal technology for some small systems. It has the advantage of being able to remove other contaminants, including nitrate, and uses a relatively short empty bed contact time. However, chromatographic peaking is a potential risk, and large volumes of brine waste are produced.
Arsenic Adsorptive Media Processes
For the arsenic Waterman Australia evaluated several technologies as candidates for best available technology for arsenic removal, including modified coagulation filtration.
Typically, coagulation filtration treatment uses the process of particle destabilisation to remove colloidal and suspended matter from the water supply. This process results in the formation of flocked particles that are removed by clarification and or filtration. The coagulants that are commonly used are aluminium or iron salts that form particulates that attach to the suspended or colloidal matter.
 Increasingly, polymer aluminium base blended chemicals are being used for the removal process. It is typically more expensive to install these traditional gravity coagulation filtering systems just for the purpose of treating arsenic. However, dissolved inorganic materials, such as arsenic, can be removed by altering the coagulation filtration process.
Increasingly, polymer aluminium base blended chemicals are being used for the removal process. It is typically more expensive to install these traditional gravity coagulation filtering systems just for the purpose of treating arsenic. However, dissolved inorganic materials, such as arsenic, can be removed by altering the coagulation filtration process.
Typically, if a source has arsenic three oxidation, usually chlorine is used to convert arsenic three to arsenic five, a coagulant in this case, ferric chloride is added to be oxidised, producing iron hydroxide particles. The hydroxides and arsenic laden water are held in a retention tank to allow the Co-precipitation and adsorption process to occur. The arsenic and iron precipitates are filtered from the water. In this example by a pressure filter vessel. The filters are allowed to load with the precipitates until the filter is partially loaded and then the filter is backwashed.
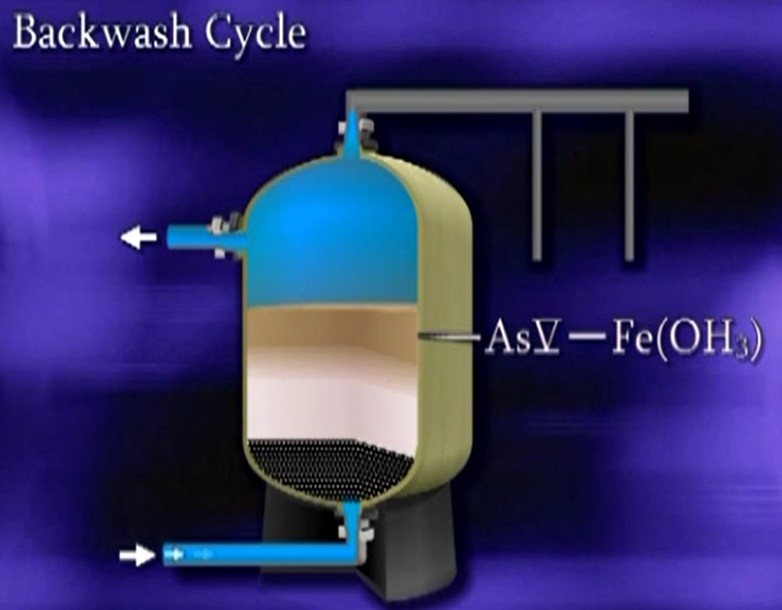 The backwash waste is directed to either sanitary sewer, or dedicated waste system. Not many surface waters have elevated arsenic concentrations that are of a concern to public water supplies. There are numerous public water systems that rely on the eldest stone River as their source water and use coagulation filtration as their water treatment process.
The backwash waste is directed to either sanitary sewer, or dedicated waste system. Not many surface waters have elevated arsenic concentrations that are of a concern to public water supplies. There are numerous public water systems that rely on the eldest stone River as their source water and use coagulation filtration as their water treatment process.
 Because it’s the surface water sources arsenic five, arsenic five is relatively easy to remove. Our treatment system here is a full conventional plant, we coagulate flocculate, sedimentation and then through filtration, we use now we use poly aluminium hydroxy chloride, which is an alum blend, proprietary alum blend prior to that we use ferric chloride. Prior to that we use now we went to a ferric chloride because of our arsenic problems and when arsenic became an issue in the early 90s.
Because it’s the surface water sources arsenic five, arsenic five is relatively easy to remove. Our treatment system here is a full conventional plant, we coagulate flocculate, sedimentation and then through filtration, we use now we use poly aluminium hydroxy chloride, which is an alum blend, proprietary alum blend prior to that we use ferric chloride. Prior to that we use now we went to a ferric chloride because of our arsenic problems and when arsenic became an issue in the early 90s.
This use alum as a coagulant. This provided an excellent full-scale comparison between aluminium and ferric based coagulants. We have treated with an aluminium sulphate in the past and polymers that we use for that. Usually, we got roughly 50% removal of the arsenic. As seen on the graph. The building’s finished water arsenic concentration was consistently below the detection level less than two micrograms per litre.
In the installed plant alum removed significantly less of the arsenic. It has since converted from ferric chloride to an aluminium polymer blend to reduce some corrosion problems attributed to ferric chloride. If the arsenic concentration is less than 15 micrograms per litre, the polymer blended coagulant works for arsenic removal, if necessary, the Waterman Australia adds a small amount of ferric chloride to aid and removal of arsenic.
Our approach here is to also use ferric chloride when we get above eight micrograms per litre in our finished water. We started adding ferric chloride.
We are testing a poly aluminium hydroxide We’re not sure if that’s the polymer, you know of choice or the coagulant of choice. we want to be able to feed a little bit of ferric to get rid of the arsenic, but we want to get that into some level that’s more controllable for pH. But I would imagine that that’s probably where we’ll be six months from now.
we’ve tested for the arsenic levels, the sludge processes that we use, sending out to a sludge drying pond have that tested for metals content, and it’s showing that we can land apply that so to this point, we haven’t had a problem with land application instead of having to take it to a landfill.
Waterman Australia is also using coagulation filtration to remove arsenic. Waterman Australia has to 140-foot-deep wells. The wells have a flow capacity of 140 gallons per minute and 160 gallons per minute.
 However, only one weld is operated at any one time with the two wells alternating on a monthly basis. The wells have about 35 micrograms per litre of arsenic, primarily arsenic three and 0.5 milligrams per litre iron.
However, only one weld is operated at any one time with the two wells alternating on a monthly basis. The wells have about 35 micrograms per litre of arsenic, primarily arsenic three and 0.5 milligrams per litre iron.
 This represents an iron to arsenic ratio of less than 20 to 1, the ratio needed for efficient arsenic removal.
This represents an iron to arsenic ratio of less than 20 to 1, the ratio needed for efficient arsenic removal.
The major components in the treatment process are
Pre chlorination
The original gas chlorination system was replaced with a liquid chlorine feed system to improve the reliability and safety of the chlorination system. This converts the arsenic three to arsenic five, and provides the oxidation of the natural and added iron ferric chloride
Coagulation
Ferric chloride is added to the water at a dose of 1.2 to 1.3 milligrams per litre to 345-gallon 42-inch diameter 72-inch-tall fibreglass contact tanks that are typically operated in parallel provide five minutes of contact time to allow the formation of iron flocks and arsenic adsorption prior to filtration.
Filtration
Filtration to pressure filters is arranged in parallel. These filtration vessels are 36 inches in diameter and 72 inches tall. Each vessel is filled with about 24 inches of macrolides media. This media is a 4060-mesh ceramic-based media.
 The macro light is underlined by one inch of Garnet sand over a slotted stainless steel under drain. Flow is controlled through the filters to 70 gallons per minute each using Flow limiting devices.
The macro light is underlined by one inch of Garnet sand over a slotted stainless steel under drain. Flow is controlled through the filters to 70 gallons per minute each using Flow limiting devices.
Automation
The system is fully automated with an operator interface, programmable logic controller and modem housed in a central control panel.
 The filters are operated at a rate of 10 gallons per minute. The pressure drop across the filters is about 15 psi. The filters are automatically backwashed at a pressure drop of 25 to 30 psi. The backwash includes an air sparge brief settling at a hydraulic wash at a flow rate of about 55 gallons per minute
The filters are operated at a rate of 10 gallons per minute. The pressure drop across the filters is about 15 psi. The filters are automatically backwashed at a pressure drop of 25 to 30 psi. The backwash includes an air sparge brief settling at a hydraulic wash at a flow rate of about 55 gallons per minute
 where the backwash starts out, it drains the tank down a little bit. And then it airs Purges and the idea of that is to stir the stuff up and kind of rub the grains together to break the stuff off. And then that settles for five minutes and then it goes into a backwash for 10 minutes.
where the backwash starts out, it drains the tank down a little bit. And then it airs Purges and the idea of that is to stir the stuff up and kind of rub the grains together to break the stuff off. And then that settles for five minutes and then it goes into a backwash for 10 minutes.
The filter to waste process is used for about five minutes prior to being returned to service. The backwash cycle is accomplished on only one filter at a time.
Chances are when we’re making backwash is we lose a little bit of a media. And then all we have to do is just add media to it. The backwash water is discharged to the sanitary sewer via newly constructed lift station. The plan produces a finished water arsenic concentration of five micrograms per litre or less and adequate margin below the new MCL of 10 micrograms per litre. Even though the system has no treatment other than chlorination prior to the demonstration project. The operator indicates that the process is easy to operate.
Arsenic Coagulating Filtration Processes
The arsenic rule has identified activated aluminium and absorptive media process as a best available technology for arsenic removal, in addition to a number of other treatment processes. In recent years adsorptive media processes using ferric based media, such as granular ferric hydroxide, have been demonstrated to provide arsenic removal. This portion will present information on two types of treatment systems.
- Iron based absorptive media
- Iron enhanced activated aluminium media.
Iron based absorptive media is being used at one of Waterman Australia arsenic treatment plant demonstration sites. Even though iron based adsorptive media was not listed as the best available technology in the arsenic rule.
 The source water is this well that produces 44 gallons per minute and operates 12 hours per day, the arsenic influent concentration is approximately 50 micrograms per litre and the pH is around seven. The treatment system consists of
The source water is this well that produces 44 gallons per minute and operates 12 hours per day, the arsenic influent concentration is approximately 50 micrograms per litre and the pH is around seven. The treatment system consists of
- Chlorination to oxidise all arsenic to arsenic five, to achieve optimal removal of arsenic
- A 50-micron bag filter to remove sediment
- Two vessels with backside ad 33 media that can be operated in either series or parallel.
When operating in series, water flows through the first vessel, and the treated water from the first vessel goes on to the second vessel.
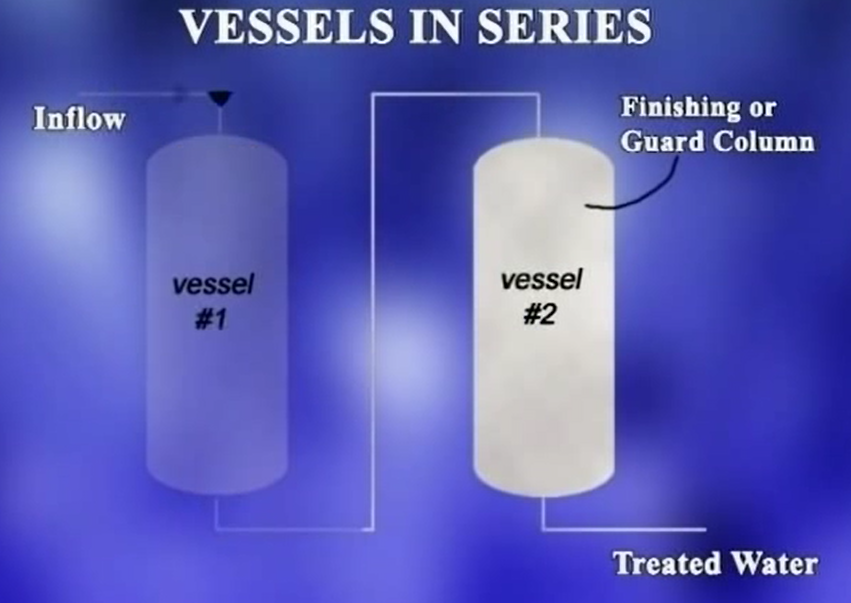 The advantage of operating in series is that the second vessel can act as a finishing column or guard column. In the event the first vessel is no longer providing adequate treatment.
The advantage of operating in series is that the second vessel can act as a finishing column or guard column. In the event the first vessel is no longer providing adequate treatment.
When operated in parallel influent water is typically split equally between the two vessels. This particular treatment system has been able to consistently reduce arsenic levels to non-detectable levels in the treated water. The media has not required replacements.
The media needs to be backwashed periodically to remove particles trapped in the media. The backwash water is discharged to this holding tank to allow settling of solids. After sufficient settling has occurred. The supernatant is recycled back through the entire treatment process. This method of residuals handling is cost effective and has worked well with this media. One important point about this system is that a pressure loss occurs as water passes through these treatment vessels.
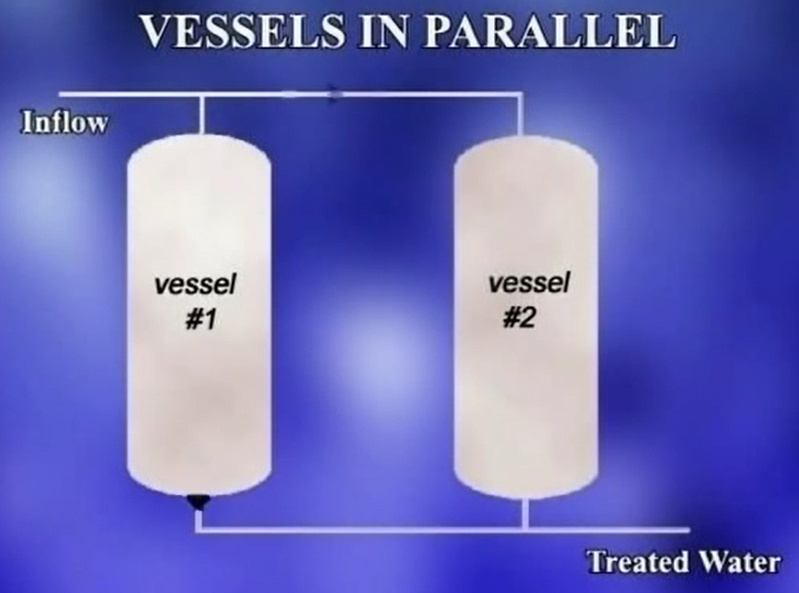 As a result, the existing well pump and treatment system can only provide 32 gallons per minute of treated water, which is less than the 44 gallons per minute production rate prior to treatment installation.
As a result, the existing well pump and treatment system can only provide 32 gallons per minute of treated water, which is less than the 44 gallons per minute production rate prior to treatment installation.
 Iron enhanced activated alumina media
Iron enhanced activated alumina media
Another Waterman Australia arsenic treatment technology demonstration site is using iron enhanced activated alumina. Activated alumina is the best available technology listed in the arsenic rule.
 The source water is this well that produces 36 gallons per minute and operates 24 hours per day. The arsenic influent concentration is approximately 30 micrograms per litre and the pH is around 7.7. The treatment system consists of
The source water is this well that produces 36 gallons per minute and operates 24 hours per day. The arsenic influent concentration is approximately 30 micrograms per litre and the pH is around 7.7. The treatment system consists of
- pH adjustment using sulfuric acid
- Chlorination
- Two vessels with iron enhanced activated aluminium media that are operated in series or parallel
pH adjustment is practised to enhance arsenic removal by this media. Initially, the pH was adjusted from the natural source water pH of 7.7 to 7.2. However, further pH reduction was conducted to obtain a pH of 6.9 to achieve better arsenic removal, chlorination is practice oxidising all arsenic to arsenic five to achieve optimal removal of arsenic.
 Although the source water arsenic is predominantly in the oxidised state of arsenic five, this treatment system has been able to consistently reduce arsenic levels to non-detectable levels in the treated water. The media has been replaced once since installation in June of 2004. The media needs to be backwashed periodically and the backwash water is discharged to this holding tank to allow settling of solids. After sufficient settling has occurred, the supernatant is recycled back through a cartridge filter, and then back into the treatment vessels.
Although the source water arsenic is predominantly in the oxidised state of arsenic five, this treatment system has been able to consistently reduce arsenic levels to non-detectable levels in the treated water. The media has been replaced once since installation in June of 2004. The media needs to be backwashed periodically and the backwash water is discharged to this holding tank to allow settling of solids. After sufficient settling has occurred, the supernatant is recycled back through a cartridge filter, and then back into the treatment vessels.
Strategies involved during Arsenic removal
 Because of the many technologies currently available for arsenic treatment systems are faced with several challenges when identifying and selecting the appropriate compliance strategy for their particular situation. Some states have been very proactive in identifying and conducting outreach to systems that will be affected by the new arsenic MCL.
Because of the many technologies currently available for arsenic treatment systems are faced with several challenges when identifying and selecting the appropriate compliance strategy for their particular situation. Some states have been very proactive in identifying and conducting outreach to systems that will be affected by the new arsenic MCL.
We’ve probably conducted I would say over 75, workshops, seminars, training sessions. We’ve done them in consultation with us here our corporation commission, which is the Public Utilities oversight agency, so that we could not only talk about the technologies that were needed, but also how to adjust rates to support the debt service necessary for installation of those technologies.
Non treatment Strategies
combining sources, creating a new source, connecting to a nearby system, or renovating the arsenic-rich well to access a better aquifer. You must now begin looking into treatment possibilities if these non-treatment options are not feasible because of restrictions on water quality, water rights, or the inability of a local system to provide system maintenance.
Treatment selection process
1. Characterise source water
To find out if naturally occurring pollutants pose treatment challenges, characterising your source water is the first step. Adsorptive media techniques and ion exchange are two of the arsenic treatment methods now in use, and both are susceptible to competing pollutants in the source water.
2. Evaluate Criteria
The treatment selection process can be overwhelming due to the vast amount of information available. We hosted a vendor fair here in the state reserved a conference room and invited all the water systems that have arsenic problems that that were contained in our listing. From our original analysis and all of the vendors we sent out notices to vendors across the country and provided them with a free booth. In a captive audience of water systems so that they could count their wares to the water systems and also so that the water systems could get side by side comparisons instead of people visiting their facilities at different times. And then added in a couple blues, we had our technical engineering staff down there to provide some unbiased advice and analysis of information that was coming from the vendor booths, as long as our corporation commission to talk about rate structures and considerations in terms of getting rate adjustments and that sort of thing. And so, we’ve really done a lot.
 3. Blending raw and Treated water
3. Blending raw and Treated water
If the arsenic levels in your source water are low, perhaps between 10 and 25 micrograms per litre, you may be able to treat a portion of the raw water and blend treated water with raw water.
This mode of operation will reduce the size of required treatment processes and result in cost savings to the water system.
4. The size of treatment facility
The size of your treatment facility will be partially determined by the type of treatment chosen, the flow rate, and any blending procedures that are carried out based on the configuration of your system. And if your system has multiple entry points, you may want to evaluate the costs of multiple treatment plants versus the cost of one centralised treatment plant and piping multiple entry points.
5. Quality and Quantity of residuals
All treatment process is created residual examples of residuals are spent filter backwash that is generated when media is backwashed or regenerate streams when ion exchange resins are regenerated. The level of arsenic in the source water, the method of treatment, and the frequency of residual generation all affects the quantity and quality of residuals.
 For instance, the ion exchange resins, and these vessels are regenerated every 12 to 24 hours.
For instance, the ion exchange resins, and these vessels are regenerated every 12 to 24 hours.
6. Residual handling and disposal
It is important that you characterise your waste as to whether or not it is hazardous based on
- The toxicity
- Characteristic
- Leaching
- Procedure or TCLP test.
The volume and characteristics of your residuals will determine what handling and disposal methods are available.
Consider the anion exchange process, which was previously demonstrated as a straightforward and efficient residual management method. All residual streams produced during regeneration are directed to this temporary holding tank, where they are processed by these granular ferric hydroxide media-filled containers. These containers are periodically removed and brought off site for disposal since the media inside them absorbed the arsenic in the waste stream.
 As a result of characterising the medium and passing the TCLP test, it has been determined that the substance is not harmful. These containers’ leftover stream is routed to another holding tank.
As a result of characterising the medium and passing the TCLP test, it has been determined that the substance is not harmful. These containers’ leftover stream is routed to another holding tank.
When this storage tank is full, a pumper truck arrives to remove the liquid and dispose of it somewhere. Once more, the TCLP test was passed by this residual stream.
7. Acceptance of Emerging Technologies
It is very important that you contact the state to obtain their input on acceptable technologies. The state may also require specific pilot testing and monitoring prior to issuing final approval of your proposed treatment system. Some systems have taken an innovative approach to the treatment selection process. By requesting bids from vendors and other companies to provide treatment on a contract basis.
 We have bid these in a way that we have not specified the technology on the bids. And we have opened the bids to a number of different technologies. We are basically doing a turnkey bid, where they’re doing all the design work. All the testing required all the permitting all the start-up operations training of personnel we put the entire burden on the contractor engineers’ vendors to more or less get that project into a position of being operate consistently under contract for five years.
We have bid these in a way that we have not specified the technology on the bids. And we have opened the bids to a number of different technologies. We are basically doing a turnkey bid, where they’re doing all the design work. All the testing required all the permitting all the start-up operations training of personnel we put the entire burden on the contractor engineers’ vendors to more or less get that project into a position of being operate consistently under contract for five years.
8. Preliminary cost estimate
Once you have identified the best treatment option or options, you should develop preliminary costs. capital costs consist primarily of equipment purchase costs, land purchase, construction, and installation costs.
 Operation and maintenance costs consist primarily of electrical costs. Me year or resin replacement chemical costs labour and other system costs required to keep the treatment plant operational. These costs will probably be used to determine funding strategies and rate setting.
Operation and maintenance costs consist primarily of electrical costs. Me year or resin replacement chemical costs labour and other system costs required to keep the treatment plant operational. These costs will probably be used to determine funding strategies and rate setting.
9. Implementation and Monitoring of treatment
Once your system has been built and is running, you should have a monitoring plan approved based on state input that specifies the contaminants to be monitored and the frequency of the necessary monitoring in place. Typically, monitoring is conducted quite frequently during initial operation, and then reduced if results are acceptable.
You will want to consult the state on the possibility of using test kits as an alternative to certified laboratories performing all analysis.
Arsenic Removal Frequently Asked Questions
1) Where does arsenic come from?
In some areas of Massachusetts, arsenic (chemical symbol As) naturally occurs in the soil and bedrock. Commercial arsenic mining had place in New Hampshire during the 1800s, but since 1985, imported arsenic has been employed in American industry. The following actions could have produced residual arsenic:
- apple orchard spraying
- coal ash disposal
- use of some pressure treated wood
Only laboratory analysis can determine the presence and concentration of arsenic because it has no taste, smell, or colour when dissolved in water, even at high concentrations.
2) How can arsenic affect my health?
Arsenic ingestion can result in both chronic (long-term) and acute (short-term) health effects.
Acute effects can include:
- Nausea
- neurological effects such as numbness or burning sensations in the hands and feet
- cardiovascular effects
- vomiting
- decreased production of red and white blood cells, which may result in fatigue
Chronic effects include:
- changes in skin coloration
- skin thickening
- small corn-like growths, especially on hands and feet
3) What about bathing/showering, or other uses?
Showering, bathing, and other domestic usage are safe unless your arsenic level is beyond 500 ppb. Arsenic does not dissipate into the air or get absorbed through the skin.
4) Where can I have my well water tested for arsenic?
To use this tool, click “search” after choosing “potable” from the “Matrix” dropdown list and “arsenic” from the “analytic” box. The outcomes, together with contact details, will be displayed at the page’s bottom.
It’s possible that not all of the labs shown in the search results test water for specific people. Please get in touch with the lab(s) before scheduling a test. “Find a Certified Laboratory for Water Testing.”
5) What is the best way to remove arsenic from well water?
Reverse osmosis looks to be the most economical way to remove arsenic from a home water supply (RO). RO is comparable to atomic scale filtering. It functions by forcing water through a unique membrane.
6) Which process can remove arsenic from water?
Oxidation, coagulation-flocculation, and membrane methods are commonly used to remove arsenic species. Additionally, significant developments in the use of different nanoparticles for the cleanup of contaminated water have been made.
7) What kind of filter will remove arsenic?
Reverse osmosis, sometimes known as RO, looks to be the most economical technology for eliminating arsenic from a private water source. RO can be considered to be molecular filtration. It operates by pushing water through a distinct membrane.
8) Where is arsenic found in well water?
Water flowing through arsenic-rich rocks and soil is one of the main sources of arsenic in drinking water wells. Both human activity and natural processes like volcanic eruptions and forest fires have the potential to further release it into the environment.
9) Can you boil water to remove arsenic?
Never try to boil water to get rid of arsenic. Arsenic levels will only rise if water is heated to a boil. Arsenic may be removed from your water at home using treatment equipment.
10) What method is most commonly used for arsenic testing?
The most typical analytical technique for determining the amount of arsenic in biological materials is atomic absorption spectrophotometry (AAS) (Curatola et al. 1978; Foà et al. 1984; Johnson and Farmer 1989; Mushak et al. 1977; Norin and Vahter 1981; Sotera et al.
11) What are safety measures against arsenic?
Eat, drink, smoke, or use cosmetics outside of work. Wear the proper protection gear, such as coveralls, gloves, hard-soled boots, hats, goggles, or face shields, if there is a chance that arsenicals will come into contact with your skin.