
Water that is used in the medical device production process and pharmaceuticals needs to meet stringent standards. In pharmaceutical engineering, water is the most important solvent. Water can be used as an excipient or can be used for the reconstruction of products, during synthesis, as a cleaning agent, or during the production of the finished product. Depending upon the use, different grades of water quality are required. And, as manufacturers of pharma grade water filtration plants, we keep these requirements in mind. Moreover, Waterman Engineers Australia as an exporter offers comprehensive solutions for pharma-grade water plants, as per the client’s needs. In pharmaceutical production, there are four types of water:
- Potable water
- Purified water
- Highly purified
- Water For Injection (WFI)
Technique:
Three types of water filtration systems are used in pharmaceutical industries.
Ion Exchange Water Filtration System:
In this system the undesirable water-soluble ions and exchanging with desirable ones. In this water treatment technology, the ionic composition is altered in a desirable direction.

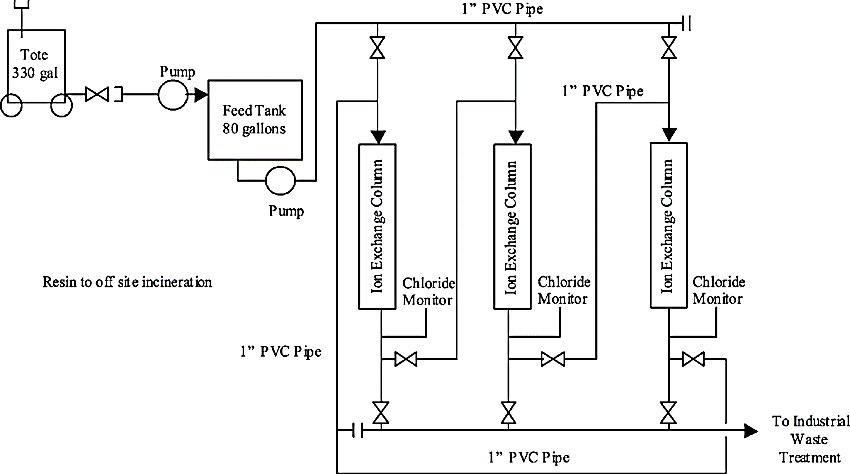
Working of Ion Exchange System:
A microporous exchange resin is the main component for ion exchange systems. This resin is suspended in a loosely held solution. Sulfonated polystyrene beds supersaturated with sodium to cover the bed surface are used for water softening. When water is passed through the resin bed, the ions attach to the resin beads and release the loosely held solution in water.
The resin must be regenerated or exchanged when it becomes saturated. The exchange resin is flushed with brine to regenerate it. Sodium ions present in the salt brine solution are exchanged with the ions and flushed out with wastewater.
Ion Exchange Water Filtration System Advantages:
- Easy to operate.
- Minimal maintenance.
- Extremely effective for removing inorganic ions from water.
- The resin used can be regenerated

Reverse Osmosis Plant Process:
In this process, natural osmotic flow is disrupted by applying pressure to the concentration solution in an osmosis process. This results in the flow of water from a more concentrated to a less concentrated solution.
How does a Reverse Osmosis Plant Work?
Reverse osmosis plants have multimedia prefilter, water softener or antiscalant dosing system, dichlorination dosing system, reverse osmosis unit with semi-permeable membranes, and ultraviolet sterilizers or post chlorination as a post-treatment.
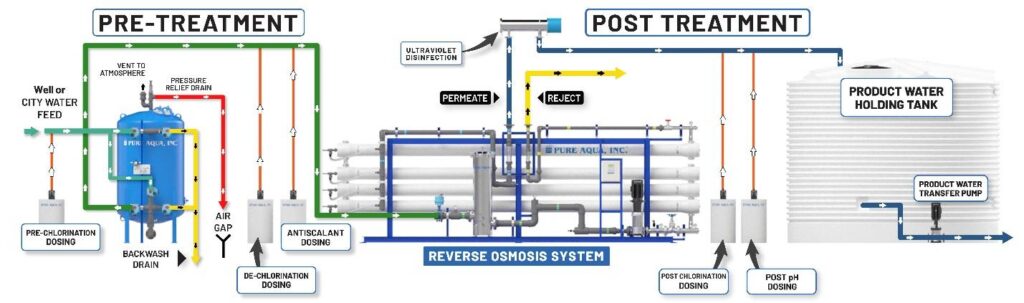
The reverse osmosis technique is applied in RO plants in which particles larger than 10 microns are removed from feed water by passing it through a multimedia prefilter. Then an antiscalant chemical is injected to prevent hardness fouling that can damage the RO membrane. Hardness, chlorine, odors, color, ion, and sulfur are removed through these pretreatment options. The minerals, impurities, and salts that weren’t removed by the pre-filter are removed by passing water through a reverse osmosis unit in which a high-pressure pump applies extreme pressure to the highly concentrated solution and removes impurities. From the low-pressure end of the membrane, fresh, potable water comes out whereas, the salts, minerals, and impurities are discharged into a drain on the other end. Lastly, to kill bacteria and other microorganisms, water is passed through a UV sterilizer.
Advantages of RO Plant for Pharmaceuticals:
- Cost-effective.
- Safe for the environment.
- Maintenance-friendly.
Pharmaceutical Water Distillation Unit:
In pharmaceutical practice, distillation is a widely used technique. As the feed water contains innumerable violent substances, the first ten to twenty percent of distillate should be discarded. Moreover, the last twenty percent should also be discarded to prevent excessive dryness which can result in the contamination of previous distillate by solid impurities. A liquid mixture containing volatile components is boiled by heat and condensation is used to recover the vapor. Additionally, as supplier we provide the best available solutions to reliably and affordably fulfil the performance requirements of these pharma grade water purifying systems.

Working of Pharma Water Distillation:
Distillation is a commonly utilised method in the profession of pharmacy. The first 10 to 20 percentage of the condensate should be removed since the supply water comprises a plethora of aggressive chemicals. Furthermore, the final 20% should be eliminated as well to avoid an inappropriate dryness that could cause solid contaminants to infiltrate the prior distillate. In order to reclaim the vapor, condensation is employed to boil an aqueous combination holding volatile components.
Pharma Distillation Plant Advantages:
- Effectively removes inorganic compounds.
- Microorganisms are also killed.
- Beneficial minerals are left behind while impurities are removed.

EDI Reverse Osmosis Plant – Electro deionization (EDI System):
A water treatment technology that is electrically driven and used, ion exchange membranes, electricity, and resins to purify water by removing ionized species.

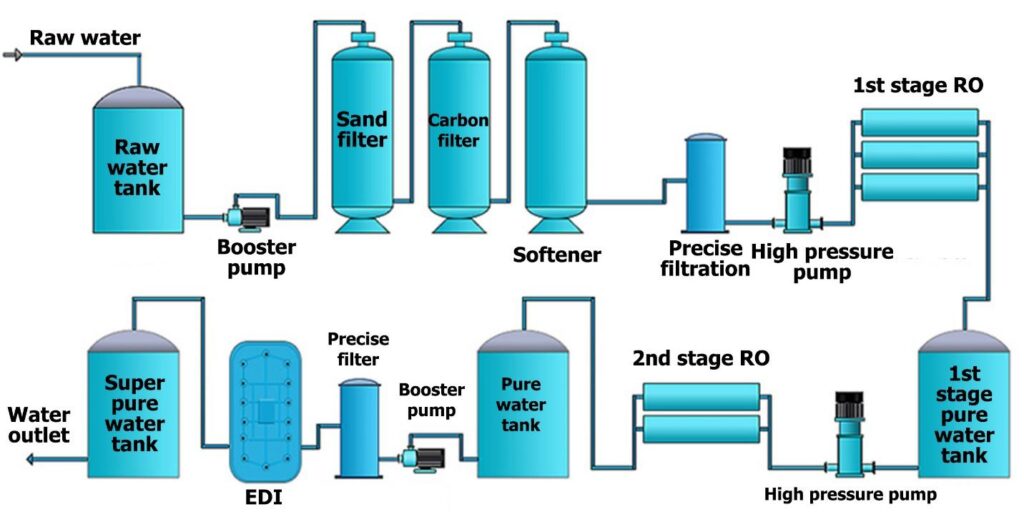
Working of EDI Reverse Osmosis System:
EDI Reverse Osmosis Plant consists of chambers that contain ion exchange resins and are separated by ion-exchange membranes. When water enters the modules, an electrical field at right angles is applied to the flow that forces ions to move through resins and across membranes. The impurities are not bound to a media and are collected in a concentrated stream which is either directed to the drain r recycled.
EDI Reverse Osmosis Plant Advantages:
- Ion exchange beds are regenerated continuously.
- The impurities don’t build up and exhaust the resin.
- Can deliver more consistent purity.
Pharma Grade Water Plants Frequently Asked Questions:
1. What is pharma grade water?
In pharmaceutical production, the water used in processing, sanitizing and as a feedstock in medicines (IV FLUIDS) must meet the strict criteria on purity standards. That water typically known as pharma grade water, with around 0 to 1 ppm TDS. Different grades of water quality are used which depends upon the end use either water is used as a medium or can be used in the manufacturing process during synthesis, as a cleaning agent or during the production of finished product. As per client’s request, two types of water are used in pharmaceutical production:
- Highly Purified
- Water for Injection
2. Major techniques to produce pharma grade water
In pharmaceutical Industries, three types of techniques are used in the form of filtration systems to produce pharma grade water:
ION Exchange Water Filtration Systems: The deplorable water-soluble ions are exchanging with the desirable ones in this system and ionic composition of water is adjust in a desirable direction.
Reverse Osmosis Plant Process: In this technique, the natural osmotic flow of water is disturbed by applying pressure to the concentration solution in an osmosis process. This results in the flow of water from a more concentrated to a less concentrated solution.
Distillation Technique: This is the extensively used technique in pharmaceutical practice.
But the most used technique for bigger quantity is RO followed by EDI.
3. How pharma grade water was being produced through water distillation process?
In order to produce pharma grade water through distillation process, at first 10 to 20 percent of the condensate should be removed since the supply water comprises a large amount of harmful chemicals, later on, the final 20% should be eliminated as well to avoid extra amount of dryness which can result in the contamination of previous distillate by solid impurities. A liquid mixture containing volatile components is boiled by heat and then vapor is recovered with the help of condensation.
4. What is ELECTRODEIONIZATION, EDI?
It is a treatment technology that is driven by electricity and used ion exchange membranes, electricity, and resins to purify water by removing ionized species from water.
5. HOW ELECTRODEIONIZATION EDI IS useful to produce pharma grade water?
It is useful to produce pharma grade water because of the following advantages:
- In this technique, ion exchange beds are regenerated continuously.
- The impurities do not build up and deteriorate the resin.
- It delivers more consistent purity.
6. HOW ELECTRO DEIONISATION WORKS?
EDI plant consists of chambers that contain ion exchange resins and are separated by ion- exchange membranes. When water enters the modules, an electrical field at right angles is applied to the flow that forces ions to move through resins and across membranes. The impurities are not bound to a media and are collected in a concentrated stream which is either directed to the drain or recycled.
7. LATEST TREND to produce pharma grade water RO + EDI
Reverse Osmosis system, EDI is the latest trend to produce pharmaceutical grade water system. The purified water and water for injection can be used for Oral liquid, finished medicine, biological preparation, etc. The GMP, FDA required water systems have activated carbon pasteurization, CIP cleaning system, distribution system ozone sterilization, distribution system pasteurization as apart of system with the most important is the EDI as a polisher to achieve TDS less than 1 ppm approximately
8. What should be conductivity for pharma grade water?
Less than 4 μS/cm
9. What should be conductivity of injection grade water?
Less than 1 μS/cm
10. How is pharmaceutical grade water produced?
Pharmaceutical grade water is produced through a multi-step process that includes filtration, reverse osmosis, distillation, and deionization. The water is then sterilized to remove any remaining microorganisms. The process is carefully monitored and controlled to ensure the water meets the required purity standards.
11. How is the quality of pharmaceutical grade water tested?
The quality of pharmaceutical grade water is tested by analysing it for various parameters such as bacteria, endotoxins, conductivity, pH, and total organic carbon (TOC). These tests are conducted at various stages during the production process to ensure that the water meets the required standards.
12. Why is it important to use pharmaceutical grade water in the manufacturing of pharmaceutical products?
The use of pharmaceutical grade water ensures that the final product is free from contaminants and impurities that could potentially harm patients. It is essential to use water of the highest purity in the production of pharmaceutical products to ensure that the final product is safe and effective for patients.
13. What is injectable water?
Injectable water is water that has been purified to a high degree of purity and is suitable for injection into the bloodstream. It is often used as a diluent for medications and other injectable solutions.
14. What is saline water?
Saline water is a sterile solution made up of water and salt (sodium chloride) in a specific concentration. Saline solutions are used for a variety of medical purposes such as hydration, electrolyte replacement, and cleaning wounds.
15. What are IV fluids?
IV fluids are solutions that are administered intravenously, meaning they are delivered directly into the bloodstream through a vein. IV fluids can be made up of a variety of substances such as water, electrolytes, and medications. They are used for a variety of medical purposes such as hydration, electrolyte replacement, and medication delivery.
16. What are some common uses of injectable water, saline water, and IV fluids?
Some common uses of injectable water, saline water, and IV fluids include:
Hydration: These solutions are used to rehydrate patients who are dehydrated or have lost fluids due to illness or injury.
Electrolyte replacement: Saline solutions and other IV fluids containing electrolytes are used to replace electrolytes that have been lost due to illness or injury.
Medication delivery: Injectable water, saline water, and IV fluids are often used as a diluent for medications that are given intravenously.
Wound cleaning: Saline solutions are often used to clean wounds and help prevent infection.
17. How are injectable water, saline water, and IV fluids prepared for use?
Injectable water, saline water, and IV fluids are prepared in a sterile environment to ensure that they are free from contaminants and impurities. They are packaged in sterile containers and are used within a specific time frame to ensure their sterility.
18. What are the side effects of injectable water, saline water, and IV fluids?
The side effects of injectable water, saline water, and IV fluids are generally mild and may include discomfort or pain at the injection site, swelling, or redness. However, in rare cases, an allergic reaction or other serious side effects may occur. It is important to consult with a healthcare professional if you experience any side effects or have any concerns about the use of these solutions.
19. What is the US Pharmacopeia (USP)?
Drugs and other healthcare goods sold in the United States must meet the criteria established by the US Pharmacopeia (USP), a non-profit organisation. The United States Pharmacopeia (USP) establishes guidelines for pharmaceutical formulations, including components, dosage forms, packaging, and testing and manufacturing procedures. These standards are used by manufacturers, regulators, and healthcare professionals to ensure the safety and efficacy of pharmaceutical products.
20. What is the British Pharmacopoeia (BP)?
The British Pharmacopoeia (BP) is a collection of standards for the quality and purity of drugs and other healthcare products in the United Kingdom. The BP sets standards for ingredients, dosage forms, and packaging, as well as for methods of testing and manufacturing. These standards are used by manufacturers, regulators, and healthcare professionals to ensure the safety and efficacy of pharmaceutical products.
21. How do the USP and BP standards compare?
The USP and BP standards are similar in many ways. Both organizations set standards for the quality, purity, and identity of drugs and other healthcare products. Both organizations also provide guidelines for testing and manufacturing methods. However, there may be some differences between the two sets of standards, particularly in the specific details of the requirements.
22. How are USP and BP standards enforced?
The USP and BP standards are not legally binding, but they are widely recognized and respected by the pharmaceutical industry, regulatory bodies, and healthcare professionals. Compliance with the standards is typically required by regulatory authorities, and products that do not meet the standards may be refused entry into the market.
23. How do manufacturers ensure compliance with USP and BP standards?
Manufacturers can ensure compliance with USP and BP standards by following the guidelines set out by these organizations, including guidelines for ingredients, dosage forms, packaging, testing, and manufacturing methods. They should also conduct regular testing of their products to ensure that they meet the standards.
24. What is the reverse osmosis process for manufacturing pharmaceutical-grade water?
During reverse osmosis, water is pushed across a semi-permeable membrane in order to filter out contaminants and dissolved solids. The water that passes through the membrane is considered to be of high purity and is suitable for use in the manufacture of pharmaceutical products.
25. What is the distillation process for manufacturing pharmaceutical-grade water?
Distillation is a process in which water is heated until it turns into steam, which is then condensed back into water. The condensed water is considered to be of high purity and is suitable for use in the manufacture of pharmaceutical products. Distillation can remove impurities such as bacteria, dissolved solids, and dissolved gases.
26. What is the deionization process for manufacturing pharmaceutical-grade water?
Deionization is a process in which water is passed through a bed of ion-exchange resin, which removes ions such as sodium, chloride, and other dissolved minerals. The water that passes through the resin is considered to be of high purity and is suitable for use in the manufacture of pharmaceutical products.
27. Are there any other methods for manufacturing pharmaceutical-grade water?
Yes, there are other methods for manufacturing pharmaceutical-grade water, such as ultrafiltration, microfiltration, and ultraviolet (UV) treatment. These methods can also be used to purify water to a high degree of purity and suitable for use in the manufacture of pharmaceutical products.
28. What are the advantages and disadvantages of each method?
Each method has its own advantages and disadvantages. Reverse osmosis, for instance, is thought to be one of the best techniques for purifying water, However, it can be expensive and demands a lot of power. Distillation is often regarded as a highly dependable approach to water purification, despite its high upfront cost and high energy demand. Deionization is considered to be a cost-effective method for purifying water, but it may not remove all impurities. Other methods such as ultrafiltration, microfiltration, and UV treatment also have their own advantages and disadvantages.
29. How is the quality of pharmaceutical-grade water ensured?
The quality of pharmaceutical-grade water is ensured through regular testing to ensure that it meets the standards set by regulatory authorities such as USP and BP. In addition, manufacturers should conduct regular testing of their water to ensure that it meets the standards and that it is free from contaminants.
30. Why was the method of producing pharmaceutical-grade water changed from distillation to RO + EDI?
The method of producing pharmaceutical-grade water was changed from distillation to RO + EDI because it is more efficient and cost-effective. Distillation required a significant amount of energy and was relatively costly, while RO + EDI is more energy-efficient and cost-effective. Additionally, RO + EDI is a more efficient method for removing impurities and dissolved ions, which results in a higher purity of water.
31. How does RO + EDI produce water of higher purity compared to distillation and DI + MBDI?
RO + EDI produces water of higher purity compared to distillation and DI + MBDI because it uses a combination of two processes. Reverse osmosis removes impurities and dissolved solids, while electro-deionization removes dissolved ions such as sodium and chloride. This two-step process results in a higher purity of water than distillation or DI + MBDI alone.
32. Are there any disadvantages of using RO + EDI to produce pharmaceutical-grade water?
Some of the disadvantages of using RO + EDI to produce pharmaceutical-grade water include the need for regular maintenance and replacement of the membranes, as well as the need for pre-treatment of the water to protect the membranes from fouling. Additionally, the method requires a significant amount of energy. However, the benefits of producing water of higher purity and cost-effectiveness make it a widely used method.
33. What is EDI?
EDI stands for Electro-Deionization. Dissolved ions like sodium and chloride are filtered out of the water by passing it through an ion-exchange membrane.
34. What are the advantages of using EDI to produce pharmaceutical-grade water?
Some of the advantages of using EDI to produce pharmaceutical-grade water include:
- High purity water production
- Low chemical consumption
- Low operation and maintenance cost
- No waste liquid or chemical regeneration
- No environmental pollution
- Compact design
35. What are the disadvantages of using EDI to produce pharmaceutical-grade water?
Some of the disadvantages of using EDI to produce pharmaceutical-grade water include:
- High initial investment.
- Requires electricity to run.
- The need for regular maintenance and replacement of the ion-exchange membranes.
36. How does EDI work to produce pharmaceutical-grade water?
EDI works by passing water through a series of ion-exchange membranes that are alternately charged with a positive and negative voltage. As the water passes through the membranes, the dissolved ions in the water are attracted to the opposite charge on the membrane and are removed from the water. The ions are then replaced with hydrogen and hydroxyl ions, which are generated by a power supply connected to the EDI module. The water that passes through the EDI module is considered to be of high purity and is suitable for use in the manufacture of pharmaceutical products.
37. What are the key components of an EDI system?
The key components of an EDI system include:
- Ion-exchange membranes.
- Anode and cathode compartments.
- A power supply to generate the electrical charge.
- A water flow control system.
- A monitoring and control system to monitor and regulate the system's performance.
38. How does EDI compare to other water purification methods?
EDI is considered to be a more efficient and cost-effective method for producing high-purity water compared to traditional methods such as distillation and mixed-bed ion-exchange. EDI requires less maintenance and chemicals compared to other methods and does not produce any waste liquid. However, EDI requires electricity to run and has a higher initial investment cost.

