
Recovery of Caustic Soda (NaOH) for Textile Effluents:
Caustic is highly alkaline in nature and hygroscopic properties. Caustic soda is also known as 'lye' and it is a white, solid ionic compound consisting of sodium (Na+) and hydroxide ions (OH–). When it is dissolved in water, it forms high pH solution. Caustic solutions have the capability to dissolve organic matter hydrocarbons, and as such chemical. It is widely used in the market to clean process equipment and for other applications.
Caustic is used in many industries in many applications. The biggest users of caustic are the pulp, paper, and textile industry. The Pulp, paper industry caustic is used to purify fibres, cellulosic fibres by removing dissolving all kinds of organic contaminants. In the textile industry, similar application of removing contaminations from rayon fibres before it go to the fabric manufacturing and mercerization.
The mercerization process is charged with caustic is used for treatment of cotton fabric to improve the lustre of the fabric and to improve the affinity to dyes and these are in all of these applications very high concentrated of caustic is used. Uses of Caustic are in the food dairy and beverage industry where caustic is used to clean equipment to make sure that the process equipment is clean for the next batch. In pharmaceutical industry, caustic is used for element to high value components materials and for regeneration of residence in various applications.
In most cases caustic is simply going to drain to the wastewater treatment plant to be treated as part of the overall wastewater of the plant. Caustic is an expensive chemical it's the price varies depending on manufacturing and production, but it's overall between 300 to 900$ per tonne of solid 100% caustic. So, recovering of caustic before it goes to the drain and before it goes to the wastewater treatment plant can save a lot of money to the user. It also saves water energy and overall, the cost saving for the recovery of caustic is to the manufacturer is very high. Also, as exporters, our plants are highly desired across a variety of sectors.
Caustic Soda (NaOH) Recovery Process

It is a caustic when it goes to the wastewater treatment plant it increases the pH of the wastewater often this makes difficult wastewater treatment process. So, before caustic is sent to the wastewater treatment plant it needs to be neutralised and by recovering the caustic before it goes to the wastewater not only the caustic is being saved, but also the acid consumption for neutralisation is being reduced. In addition, the overall carbon footprint of the plant is reduced and there are savings by reducing the flows to the wastewater treatment. It's interesting because the effluent that recovered caustic from the membranes is better than the raw caustic that buy originally. Overall, the reduction from the caustic of organic matter is almost complete the caustic is clear, and the caustic is at the same concentration of what was fed into the membered system.
There is a complete solution, also a family of Caustic Recovery systems in market for recovering the caustic from mercerization. Available membrane is based on the central product, this is a nanofiltration membrane that not only has an optimal separation property to pass the cost they can retain all the contamination, but it also has the stability that is required to withstand these high pH conditions and often also high temperature conditions. This membrane is a spiral bound element around it. Designed system family the caustic of systems that offer solutions for small medium and large flow rates for recovery of caustic.
These are very harsh conditions. Typical caustic is used in the industry, anything between 1-20% and the temperatures can go as high as 70 degrees centigrade or 160 Fahrenheit. The combination of high concentrated caustic at high temperature or very corrosive harsh most materials fail in short time. The construction materials of the systems and membranes have to be very carefully picked in order to serve and provide long life of product in the industry. Typical life can be between 1-5 years depending on the cost the concentration and depending on what is to be removed from the cost state, but a good number is 1 to 5 years.
Smallest system typically treats around 5000 gallons per day or one cubic metre per hour. It is based on one single pressure vessel with loaded with spiral bound elements. Their largest system is designed to treat 250,000 gallons per day. For larger flow rates, as manufacturers of caustic recovery plants we can either customise the larger system or provide multiple scales of the large systems. Moreover, as a suppliers of caustic recovery plants our products are praised by customers for their dependability, durability, stellar result, and sturdy build.
As Caustic recovery plant manufacturer these automated systems operating mode varies from the small systems to the large systems, the nature of the small systems are more, they are more manual, that's basically what the client wants from a very small system, they operate in a batch mode, meaning that we have a tank it contains caustic, contaminated caustic, we run a batch mode, we treat this caustic and then clean the membrane and move on to the next day.
The medium sized systems have a lot more automation they are kind of what we defined semi automatic, they can run at both batch mode or continuous mode and the large systems are fully automated with full controls and they run in a fully continuous operating mode.
Working Principle of Caustic Recovery Plant:
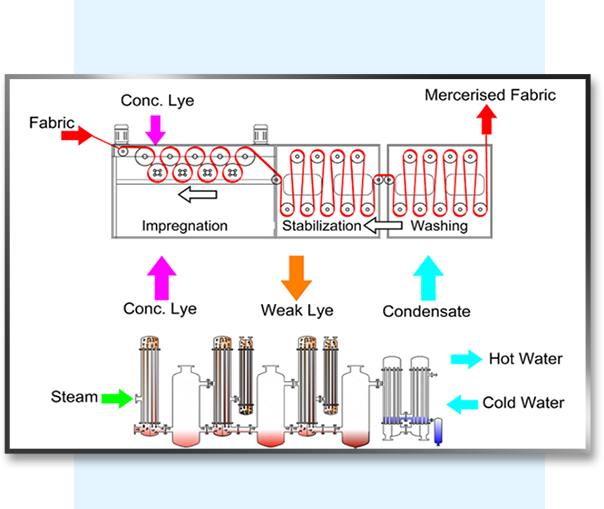
Caustic soda recovery / caustic recovery method with the use of a heat source, caustic is heated to its boiling point, and the vapours produced by boiling are used to heat the weak liquid.
The vapours formed by flashing this heated liquid at a lower pressure are then utilised again. The weak liquid is concentrated to 250-300 gpl in dry Mercerized and 450-500 gpl in wet Mercerized, which can be reused with moderate caustic loading.
Explanation of Working Caustic Recovery System:
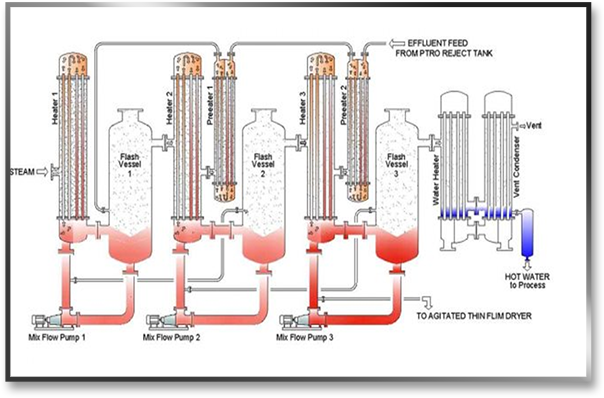
In Caustic recovery plants for textile industry, Weak Caustic lye, around 5-6 Be (40 - 50 gpl), is collected in a storage tank from the impregnation and washing chamber. The suspended particles, fluff, and other debris are then filtered in a filtration unit of the Caustic Soda Recovery Plant. The feed to CRP then runs through a series of preheaters, which use flash vapour from the condensate flashes to preheat the wash liquor, resulting in little steam loss while heating the wash liquor to its boiling point. The liquor in the tubes comes into touch with the steam in heater 1's shell side and begins to boil. The vapour liquor combination enters the flash vessel via a tangential entry, where the vapors separate and enter the 2nd stage shell.
The lower pressure developed with the help of a water ring vacuum pump causes the boiling in the successive effect. The boiling point of the liquor is reduced as a result of the decreasing pressure, and it begins to boil. Due to the pressure differential, the concentrated liquor enters the second heater and comes into indirect touch with the produced vapour, and the process is repeated. A product pump removes the concentrated product from the last step. This is then either stored or purified using a Concentrated Lye purification technique.
The vapour generated at the final stage of the caustic recovery system / caustic soda recovery plant must be condensed. Either hot water or an adiabatic evaporator system is used to accomplish this. Water at room temperature is passed through tubes of an exchanger in a hot water system, where it becomes hot due to vapour. Because this hot water is uncontaminated, it can be safely used in the process or boiler as needed.
The effluent is transported through the exchanger and sprayed in an Adiabatic Evaporator in some circumstances where the hot water created cannot be properly used. Waste heat is lost in the form of vapour, resulting in lower effective steam usage. Also gone is the annoyance of too much hot water.
Who can install this caustic recovery system?
As a Caustic recovery plant supplier, if you are user and that you purchase caustic in large quantities and you dispose caustic in large quantities to your wastewater treatment and most likely also use acid to neutralise this caustic and you use the caustic at 1% concentration or higher, most likely you're a very good candidate for caustic recovery system. Overall, you will save money on purchasing less caustic and let's not forget the environmental impact of recovering caustic. Caustic has a lot of sodium in it and disposing sodium into the environment is not a good thing. It's a global goal to reduce disposal and use of sodium. So, recovering caustic is basically improvement of environmental sustainability and if we can help the environment and save money, this is a win situation.
It Is Worth To Recover Caustic Soda:
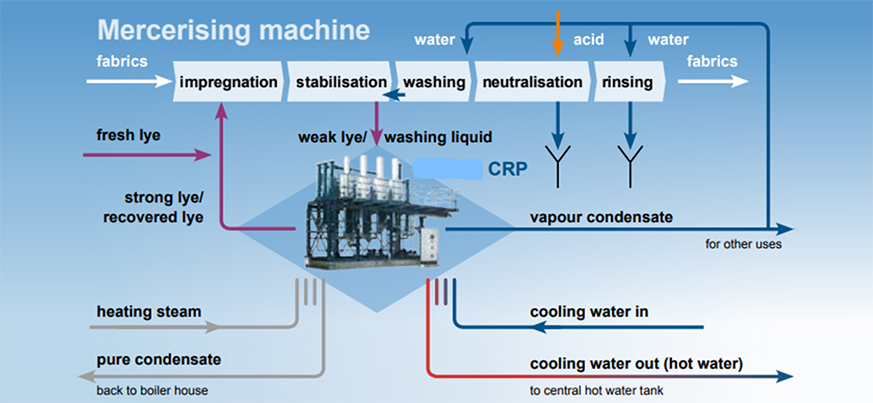
Chemicals are used in large quantities by the textile industry to prepare, clean, and colour materials. Caustic soda (NaOH) is one of the most often utilised compounds to improve the strength and shine of cellulosic materials. Cotton is dipped into a concentrated caustic soda solution and additional wetting agents under tension at room temperature during the mercerizing process. The mercerized fabric is rinsed with water at the end of the mercerizing cycle to remove any surplus caustic.
The rinse water, which is mostly weak caustic, is typically produced in large quantities, and if discharged without treatment, can result in a significant loss of caustic soda and a high consumption of acid for neutralisation in the Effluent Treatment Plant (ETP), resulting in large volumes of sludge.

Advantages of
Chemical Recovery:
- Reduced waste caustic discharge & effluent treatment costs
- Savings from reduced purchases of caustic soda
- Saving in energy cost for heating water
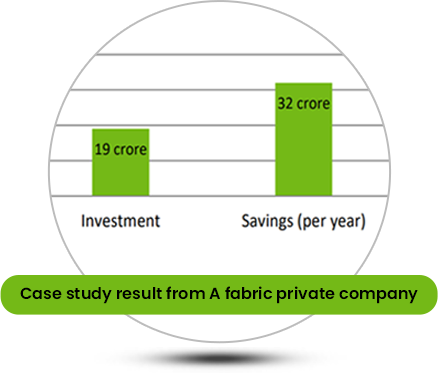
Why Caustic Recovery Plant is Necessary?
After food, cloth is the second most important need of humans. The textile industry's fundamental milestones are producing fibres and then processing them to manufacture clothing.
Because of this unavoidable need, the textile industry has developed at a quicker rate as the population and living standards have risen over time. As a result, the textile industry has established itself near countries' biggest export and import markets.
Caustic Recovery Plant Frequently Asked Questions :
What is the purpose of a Caustic Recovery Plant in textile effluents?
Textile manufacturers use the caustic recovery plant to recover the caustic soda used in mercerizing impressively. This reduces any kind of pollution as well as the cost. Mercerisation is a finishing process in the textile industry where the textile fibers are treated under tensile stress with caustic soda.
Are there different types of Caustic recovery plants available, and how do they differ?
Caustic recovery plants are designed as per user specifications. The smaller ones and the larger ones depend on the volume to be treated. The smaller ones use a manual system as they operate in batch mode. The medium-sized systems have much more automation, while the larger ones are fully automatic.
What is the working principle of the caustic recovery plant?
The caustic recovery plant uses a heat source where the caustic is heated to a boiling point, and the vapours produced by boiling are used to heat the weak liquid. The vapours formed by flashing the heated liquid at a lower pressure are then utilised again. This process is repeated until the desired concentration is obtained. After processing in the mercerizing machine, the diluted caustic soda solutions come out as a drain.
What are the advantages of using a Caustic recovery plant?
The caustic recovery plant offered by Waterman Engineers Australia offers-
- Maximum recovery of Caustic
- An energy-efficient system
- Less Maintenance is required
- Can handle capacities
- Minimum manpower is needed as there is automation as per client requirement
What is a Caustic Recovery Plant?
A Caustic Recovery Plant is normally a multiple effect evaporation plant that heats the weak lye in a heat exchanger to re-concentrate it, and when it reaches the evaporator, vapour is released due to water evaporation, which enhances its concentration.
Can caustic be harmful?
Sodium hydroxide is what we call a caustic. Workers should take care to avoid coming into contact with sodium hydroxide. The severity of harm is proportional to the quantity, duration, and intensity of the offending stimulus. It can temporarily wipe out your hair and burn your eyes, skin, and internal organs.
What consequences does caustic have?
Shortness of breath, drooling, difficulty swallowing, vomiting, and vomiting blood are all possible symptoms. A person may experience very low blood pressure (shock), trouble breathing, or chest pain in severe situations involving potent caustics, which could result in death.
What kind of danger does caustic pose?
Category 1A Hazard has been assigned to this substance (irreversible effects on the skin). This substance is not considered to be a respiratory sensitizer when inhaled.
What occurs if you inhale caustic?
Inhaling caustic substances can irritate the stomach, lungs, nose, and throat. Even burns to the airways can happen from really strong exposures. Inhaling caustics can cause discomfort in the throat and/or nose.
What does caustic serve as?
Numerous applications exist for Caustic Soda, also known as Sodium Hydroxide, including drain cleaning and soapmaking. The primary uses of caustic soda include cleaning drain pipes, clearing clogged drains, removing built-up grease from ovens, and producing soap and detergents.
What purpose does NaOH serve in textiles?
It is also used in the production of dyes, the processing of cotton fabric, the laundering and bleaching of garments, and the extraction of metals using electrolysis.
What function does caustic soda serve in the textile industry?
Additionally, mercerizing, a technique for enhancing the strength, gloss, and dye affinity of the cloth, uses caustic soda. During this procedure, caustic soda is given to the fabric to create fibre swelling, which improves the fabric's characteristics.
Why does dyeing utilise caustic soda?
It is employed to prepare fabric for dye absorption. It is a key or important chemical in the process of dyeing fabric that holds the dye and hydrosulfite in place.
What role does soda play in the dyeing process?
By increasing the pH of the dye bath, soda ash works. By adding soda ash, the solution becomes less acidic and more basic. The fabric molecules are activated by the higher pH level, allowing for improved colour absorption. As a result, the colours are more vibrant and durable.
What kind of chemical is employed in mercerization?
The alkaline treatment, also called mercerization, is a chemical process in which natural fibres are soaked in a solution of sodium hydroxide (NaOH) for a certain amount of time and at a certain temperature.
How much caustic soda is used in the mercerization process?
At a temperature of 15 to 18 °C, cotton is subjected to the mercerization process, which involves treating it with concentrated solutions of caustic soda (20 to 30 % by weight).
How long must cotton fabrics be subjected to caustic treatment in order to mercerize them?
The procedure involves briefly submerging the yarn or fibre in a sodium hydroxide solution (caustic soda)-typically for less than four minutes. The sodium hydroxide is subsequently neutralised by treating the substance with either acid or water.
What distinguishes mercerization from causticization?
In two ways, causticization and mercerization are different from one another: tension is not applied during causticization, and the concentration of caustic soda used is lower than it is during mercerization. The procedure is typically carried out either after scouring or after bleaching, with the specific goal of enhancing the fabric's ability to absorb pigment.
Which elements influence mercerization?
- Tension.
- Temperature.
- A concentration of caustic soda.
- Treatment duration.
- Washable state.
- Use a wetting agent.
- Tempo of drying
- Yarn construction.
Is mercerization a form of finish?
For cellulose fabrics and yarns, primarily cotton and flax, mercerization is a textile finishing process that increases dye uptake and rip strength, lowers shrinkage, and gives the fabric a silky shine.
What function does mercerization play in textiles?
One of the most significant methods for enhancing cotton's shine, hand, and other qualities is mercerization, which uses a powerful caustic alkaline solution. It gives the fibre more gloss and boosts its hygroscopicity, strength, and dye affinity.
What kinds of mercerization are there?
- Mercerization in grey (Knitted fabric before dyeing)
- Mercerization of textiles (Yarn dyed fabric)
- Cuff and collar mercerization
What does cotton mercerization entail?
Threads are pulled through a 20 percent caustic soda cold solution to create mercerized cotton. The fibres enlarge as a result, and when compared to a non-mercerized equivalent, this increases tensile strength by around 12%. Mercerized cotton is widely used in both domestic and industrial production.
How can you tell whether a fabric has been mercerized?
Mercerized cotton will turn blueish-black when exposed to a solution containing 20 g of iodine in 100 ml of potassium iodide for three seconds, but un-mercerized cotton will remain white.
Are garments made of mercerized cotton good?
It has the most elegant cotton we've seen, with a shine that stays put even after multiple washes. Still, mercerized cotton shines because of its enhanced durability and colourfastness. So, it's incredibly sturdy in every way imaginable. We use it for our go-to tees and tops because they can last through multiple seasons with minimal wear and tear.
Is mercerized clothing secure?
Mercerized fibres have undergone a non-toxic process that involves submerging them under tension in a potent lye solution before washing it out. This delivers great colourfastness while also permanently enhancing the fabric's strength, absorbency, and look.
Describe weak lye.
The cloth was treated with water after a mercerizing cycle to remove extra caustic lye from the fiber's surface. As a result of water washing, the caustic lye concentration keeps dropping. Because of its concentration, which is between 6 and 7 percent, this solution is known as weak lye.
Can caustics be flushed down the toilet?
Caustic soda should not be used to unclog your drains. The first is that, because these substances are corrosive, they can eat through obstructions while also causing harm to your pipes and drainage system.
What pH range does lye fall into?
With a pH of roughly 14, sodium hydroxide lye is at the very top of the pH scale. The pH level of the soap might approach 11–14 when too much lye is used, which is higher than the normal range of 9–10.
What is the ideal lye to water ratio?
Do not forget that lye must be dissolved and diluted in distilled water in order to produce the lye solution. Thirty percent lye and seventy percent water are a typical dilution ratio. In other words, the lye combination is made up of 2.3 parts water and 1 part lye overall. The mixture becomes more lye concentrated as the lye to water ratio rises.
What caustic solution should I use for cleaning?
It is also known as sodium hydroxide (NaOH) or caustic soda. Extremely acidic alkali, typically used at concentrations between 0.5% and 2.0%. Concentrations as high as 4% can be used to clean extremely grimy surfaces.
What is the sole material that is appropriate for usage with caustics?
The most frequently considered metals and alloys for usage in caustic soda include nickel and high-nickel alloys, carbon steel, and stainless steels.
How is pH impacted by caustic?
Caustic soda, also known as sodium hydroxide (NaOH), raises the pH of water without introducing the calcium ions necessary for calcium carbonate precipitation. As a result, compared to lime, caustic soda should have a lower potential for precipitating calcium carbonate for a given pH adjustment.
What is caustic's conductivity?
Caustic generally has a conductivity of 40 mS/cm, whereas water has a conductivity of >1 mS/cm. The conductivity cell recognises this difference and triggers an in-line three-way valve to open, diverting the water to the effluent plant.

