Various Chemicals Used in Effluent Treatment Plants
Chemical treatment solutions or effluent treatment plants are for advanced treatment of wastewater, removal of nutrient and disinfection or other pollutant. The secondary effluent that we receive from the conventional wastewater treatment systems, the secondary biological systems in which the solids are basically removed in two stages in conventional system are treated in tertiary effluent treatment portion where we used different chemicals to remove pollutant.
One at the primary sedimentation system which removes floating materials and grit in fact a lot of inorganic solids grits those kinds of things are removed in the grit, but in the main treatment chain, the primary sedimentation removes a large portion of the suspended materials whether they are organic or inorganic.
 So, that removes the microorganisms which grow in the biological units before secondary settling. Whether it’s a trickling filter or its activities sludge process, so, that biomass is settled in the secondary sedimentation basin.
So, that removes the microorganisms which grow in the biological units before secondary settling. Whether it’s a trickling filter or its activities sludge process, so, that biomass is settled in the secondary sedimentation basin.
Still there are some solids particularly the fine sediments they do not settle in either primary or secondary sedimentation basin because of their smaller sizes or other things and particularly in the secondary because there is a little gas production as well. So, many times the solids are attached with the gas molecules which makes them lighter, and they do not settle.
This could include even the bacteria also some of the bacteria which is not removed in the secondary sedimentation that also comes in the effluent of the conventional effluent wastewater treatment systems.
They need to be removed by a further sedimentation and or kind of either sedimentation or filtration or a combination of both sedimentation and filtration with the addition of coagulant.
So, kind of simple coagulation flocculation process can be employed for that, where a coagulant is added and that coagulant then kind of initiate the formation of flocks, since the flocks are bigger particle. They agglomerate, and they settle in the sedimentation basin even if they are not settling in the sedimentation basin. We may need to go for a filtration unit, where these flocks or micro flocks can be settled.
Many times, even the coagulation is not needed and a simple sand filtration kind of process right after the conventional secondary settling tank could help in removal of these solids.
Coagulation and Flocculation Method
If we pass the water to these effluent recycling systems, we can achieve the removal of these fine sediment particles these fine suspended particles just through a simple filtration. However, if they’re too small to basically be trapped even with the filter of the appropriate size, so we may need to coagulate and flocculate them beforehand using some coagulation and flocculation add. So, usually in-organic coagulants are used.
- In-organic coagulants such as salts of iron and aluminium. So, Alum, ferric chloride, ferric sulphate those kinds of things can be used.
- However, at times the blending organic and inorganic, both type of coagulant can be more helpful in fact and at times people use both inorganic and organic cobbling together.
Both have kind of a different mechanism the inorganic coagulants facilitate more of the sweet flock formation particularly if there is a significant dose of the coagulant. So, like if we add a lot of iron ore ferric chloride something like that, this will settle as a ferric hydroxide also a large mass of ferric hydroxide is generated and when this mass settles, so, its kind of all the small particles in between settles along with this.
The organic coagulants will eventually help in the sludge generation part. So, if we see the Alum, lime or Iron salts are the chemicals that are used in the effluent treatment plant or effluent recycling plants to remove these fine solids.
So, with the chemicals the smaller particles clump or kind of flock together into the large mass and then this large mass settles out in the sedimentation tank or can be retained in the filtration basin.
So, if we see the different coagulant like polyamine, melamine formaldehyde, all these things whereas, inorganic coagulants include aluminium sulphate, aluminium chloride, poly aluminium chloride (PAC), is getting quite popular these days then ferric sulphate and ferric chloride So, particularly the sorts of Iron and Alum that way so, these are the methods to remove.
The process is simple just add coagulant make a rapid mix so, as we do in a conventional say water treatment systems, we can in a rapid mix we add little coagulant and then allow it to settle for some time or pass it through filter these coagulants can be added in secondary treatment stage as well.
 So, the little coagulant could be added in the effluent coming from the aeration basin where the secondary sedimentation tank itself we can achieve some flocculation and the removal of these solids.
So, the little coagulant could be added in the effluent coming from the aeration basin where the secondary sedimentation tank itself we can achieve some flocculation and the removal of these solids.
Chemical Co-precipitation of Dissolved metal ions
As Arsenic removal plants manufacturer, there are chemical precipitation methods used for removal of the dissolved metal ions. The metals or metal ions which are dissolved in the water, their solubility depends on the PH.
As if we basically make the pH towards the alkaline range normally what we see that the solubility of these metal ions is reduced drastically. If you bring the pH around 10 solubility of nickel which usually like earlier which at a neutral pH or around 7 or 8 is more than 100 milligrams per litre will reduce down to say less than 0.001 milligrams per litre or that way 1 microgram per litre.
 Once the solubility of these metals reduces, they precipitate in the form of metal hydroxides. So, copper at around pH nine has a solubility of less than 0.1 microgram per litre, so, that way if we adjust the pH accordingly, if most of the metals actually we know that the solubility increases at lower PH. So, if we increase the pH by adding lime or those kinds of things, what happens that as the pH increases these metals precipitate in the form of their hydroxides.
Once the solubility of these metals reduces, they precipitate in the form of metal hydroxides. So, copper at around pH nine has a solubility of less than 0.1 microgram per litre, so, that way if we adjust the pH accordingly, if most of the metals actually we know that the solubility increases at lower PH. So, if we increase the pH by adding lime or those kinds of things, what happens that as the pH increases these metals precipitate in the form of their hydroxides.
That way we can reduce these matters by just simple chemical precipitation. So, copper, nickel, Iron, many of such metals actually can be reduced by the chemical precipitation.
- There are several other metals like for say arsenic. These kinds of metals do not precipitate in the form of their hydroxides usually, but they can actually be co-precipitated along with the precipitate of other metals particularly the Alum and iron salts. If there is Iron coagulant is added it will form ferric hydroxide precipitates.
- Now, this ferric hydroxide can adsorb the arsenic species is arsenic compounds on their surface through either chemical interaction or through simple adsorption process like Iron is a pretty good adsorbent for arsenic and many of the field arsenic removal methods are basically derive based on the ability of Iron to adsorb the arsenic.
For Arsenic removal from waste water many methods suggest that we add Iron coagulant and form the precipitates of Iron and these precipitates will then adsorb the arsenic.
So, that way the removal of such metals for say arsenic is through Co-precipitation they do not precipitate as a hydroxide, but they get adsorbed on a precipitate of some other. Iron or aluminium hydroxide how they are removed from the system because when they adsorb on the precipitate and those precipitates settle, along with them, these metals also settle. So, for removal through Coprecipitation we must add a coagulant, and which will generate micro flocks and on the surface of these micro flocks, these particles will settle down.
During the coagulation flocculation process, many micro particles and charge ants will be attached to these flocks and then subsequently we can basically process them in a simple filtration unit or sedimentation unit if they can remove by sedimentation, sedimentation is fine if not we can go for filtration. These kinds of particles if you want to reuse the water, we go for high order filtration like micro filtration or that kind of thing.
These micro flocks will be separated from the effluent treated water and that way the kind of the water that comes will be divided off these metals as well.
Advance oxidation process
Advanced oxidation processes also there which targets the removal of various compounds which have higher chemical stability or low biodegradability. The refractory organic compounds, which we discussed that can be removed through adsorption or those kinds of things.
Adsorption is a process of removal or phasing out them, but advanced oxidation processes are the one where they can be decomposed and degrade to nontoxic elements only thing is this advanced oxidation process (AOP) are energy intensive processes. So, these compounds which have low biodegradability are kind of complex structure are attempted to be completely mineralized.
The complete mineralization of these pollutants to CO2 water and various inorganic compounds are targeted and if it is not being completely mineralized or the complete minimization is not being achieved is still it is attempted that at least their transformation into the more innocuous products is ensured, so, that the toxicity or the threat of these pollutants is reduced in the water.
Advanced oxidation processes are used as a pre-treatment to biological processes. Because even the partial like if you are having a compound which is very difficult to biodegrade with a complex structural compound say some ring compound and then attached has a lot of functional groups there.
Now, this kind of compound if you want to degrade by the bacteria, probably because of its non-biodegradable nature bacteria will not be able to decompose it then what to do. Other processes either you adsorbed, but it is still being phased out the compound is still there, when you regenerate that adsorbent or you are regenerating that or if you are removing it through RO or that kind of system membrane processes, then that is again coming into the concentrate. So, problems still are persisting.
Other option is to chemically or this through advanced oxidation process you degrade that, but there would be enormous cost. So, what can be done like how this chemical process or how these advanced oxidation process decomposes or degrade those compounds is by producing the hydroxyl radicals OH radical.
When these OH radical is kind of highly reactive, so this can attack the compound and break it down. This breakdown is through various intermediate processes. It’s not that it’s like it attack the compound and it completely mineralized it immediately. It doesn’t happen that way there is a pathway for this.
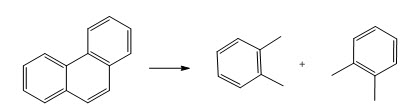 Now, when this radical goes maybe you see the breaking of just one bond or maybe this functional group is eased out or maybe this part is separated. So, you may actually see two compounds and then other compound like above.
Now, when this radical goes maybe you see the breaking of just one bond or maybe this functional group is eased out or maybe this part is separated. So, you may actually see two compounds and then other compound like above.
- So, that way, this is a sequential step where this degradation or decomposition takes place in the various intermediates, the biological degradation also takes place in similar way.
- So, what’s the point here that this compound may not be biodegradable, but once it is broken down to probably this becomes biodegradable. So, we may not need to basically completely mineralized using advanced oxidation process which are quite energy intensive.
We can initiate the process we can kind of do couple of steps where the non-biodegradable organic compounds are converted to biodegradable organic compounds. So, when they convert it to the biodegradable intermediates, we can stop the process there and from there onwards we can go for the biological treatment systems. where these compounds will then be degraded completely.
So, that is also can be done and advanced oxidation processes are able to do that and that’s why many times they are used as a pre-treatment to biological process. So, like particularly in the industries, if you are having very complex waste, so very high COD, but very low BOD that means there are a lot of organics, but non degradable.
Let’s say donate that water. So, donation does is kind of reduces the like do some initial oxidation step and as a result, those non-biodegradable compound converts to the biodegradable so, what we may see that the COD is reduced, but large part of COD has been converted to BOD. So, BOD might see in fact in increasing the BOD in such cases.
So, that way the advanced oxidation process works. So, this advanced oxidation process essentially targets the oxidation through this highly reactive OH radical. How these OH radical is produced, there are different methods for that. So, these OH radical could be produced using different region system, which could include like photochemical degradation process, UV/ O3, UV/H2O or photocatalysis TiO2/UV or photo-Fenton reactive or chemical oxidation process where ozone (O3), ozone, hydrogen peroxide (O3/H2O2) or hydrogen peroxide and Iron (H2O2/Fe2) can be used.
 So, eventually all these advanced oxidation processes will somehow generate the OH– radical and this OH– radical will react with the pollutant and convert them to CO2, H2O and inorganic ions. So, that is what is done in the advanced oxidation process. However, the production of OH radical through these processes is quite a cumbersome task and may need significant amount of energy at times.
So, eventually all these advanced oxidation processes will somehow generate the OH– radical and this OH– radical will react with the pollutant and convert them to CO2, H2O and inorganic ions. So, that is what is done in the advanced oxidation process. However, the production of OH radical through these processes is quite a cumbersome task and may need significant amount of energy at times.
If we see the different advanced oxidation processes. We have various homogeneous process and heterogeneous process where catalytic oxidation photocatalytic oxidation or Hydrogenous photocatalysis kind of produce OH radicals among homogeneous there are a few methods which does not need too much of energy rather works on a chemical these things are like Ozone/H2O2, Ozone in alkaline medium or H2O2 and catalyst.
 Whereas there are processes which needs energy in the form of ultraviolet radiation or ultrasound energy or electrical energy. So, Ozone/UV, H2O2/UV ozone/H2O or UV/photo-Fenton or on an ultrasound energy, H2O2 an ultrasound energy or like electro-Fenton process, anodic oxidation.
Whereas there are processes which needs energy in the form of ultraviolet radiation or ultrasound energy or electrical energy. So, Ozone/UV, H2O2/UV ozone/H2O or UV/photo-Fenton or on an ultrasound energy, H2O2 an ultrasound energy or like electro-Fenton process, anodic oxidation.
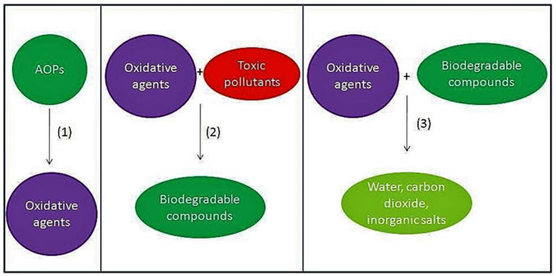
There are all these processes. So, these different processes eventually target to generate the OH radical which can then be used for the process. So, how it is done the process is pretty simple. The first step is that it actually produces the oxidative agent which is OH radical now, this oxidative agent reacts with the toxic pollutant and convert them to biodegradable organic compounds.
This oxidative agent further will react with the biodegradable compounds and convert them to the mineral stage. we are trying to use as a pre-treatment purpose, we actually end the process only So, this is for pre-treatment. But when we are willing to go for complete treatment, then we’ll have to kind of take the process further up to this point.
 Disinfection
Disinfection
We can achieve disinfection through RO system. We can achieve disinfection through the origination or advanced oxidation processes as well.
However, if we are not like these processes are typically not used only for disinfection, if there are other things then we can use these and we can achieve disinfection also, but if we are just targeting disinfection based on our end use of the effluentor based on the requirement of the national regulatory agencies the discharge standards whatsoever are there.
If we need to remove the pathogens from secondary treated effluent without probably going for various other things, we can use the some of the conventional disinfection approaches for treating the water and we can disinfect the water by chlorination, by ozonation, by using ultraviolet light those kinds of things can be used.
Common Disinfectants
- NaOCl
- Ca(OCl)2
- Cl2 gas
- Chloramines
- Ozone
- UV irradiation
If we see the common disinfectant that are used, than chlorine is by far the most popular and most common, which can be used in the form of chlorine gas, which can be used in the form of hypochlorite sodium or calcium hypochlorite or could be used in the form of chloramines. Chloramines are generally like have lesser disinfection abilities as opposed to the chlorine gas or this hypochlorite which eventually convert to so. What happens when water reacts with the chlorine it eventually produces HOCl, and HCl.
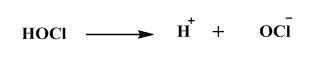
![]() So, this HOCl, which is called hypochlorous acid has quite high disinfection abilities and this is actually this HOCl depends on the PH so it can be broken down to H+ and OCl–.
So, this HOCl, which is called hypochlorous acid has quite high disinfection abilities and this is actually this HOCl depends on the PH so it can be broken down to H+ and OCl–.
 This OCl– ions, which is called hypochlorite ions, so these HCl and OCl– both have disinfection abilities both can both are stronger oxidant and can actually oxidise because chlorine become the electron acceptor that when can oxidise the microbial components leading to the death or decay of the biomass or microorganisms that way.
This OCl– ions, which is called hypochlorite ions, so these HCl and OCl– both have disinfection abilities both can both are stronger oxidant and can actually oxidise because chlorine become the electron acceptor that when can oxidise the microbial components leading to the death or decay of the biomass or microorganisms that way.
There is chlorine dioxide can also be used, which is very effective, but kind of it’s difficult it needs to be produced at site which needs skilled that way, it is not like. It’s not that popular to use chlorine dioxide in the field.
Then there are ozone and UV radiations. So, that ozone is again is as strong oxidants which kind of have the disinfection abilities as well and UV. So, bacteria, cell walls adsorb UV light at some radiations around they absorb UV light. So, if we put the UV light off those radiations those energy is absorbed by the microorganism’s cell walls and then they that is disrupted and as a result micro-organisms become dead.
 UV is getting more and more popular actually nowadays. So, chlorine has a risk particularly in the wastewater system, because if the complete removal of the organics has not been ensured, so, there is possibility of finding disinfection by-products DBPs which could have little potential toxic impact. So that’s what is one of the issues with chlorine but quite effective and most popular undoubtedly, for the disinfection purpose.
UV is getting more and more popular actually nowadays. So, chlorine has a risk particularly in the wastewater system, because if the complete removal of the organics has not been ensured, so, there is possibility of finding disinfection by-products DBPs which could have little potential toxic impact. So that’s what is one of the issues with chlorine but quite effective and most popular undoubtedly, for the disinfection purpose.
If the disinfection it normally involves the injection of chlorine solution at the head of the chlorine contact basin. These will depend upon the strength of the wastewater another factor but 5 to 15 milligrams per litre doses are usually normal.
The time requirement or the concentration of the disinfectant will both govern the degree of disinfection. So, there is kind of like concentration into time which is called CT. So, CT is used for seeing the degree of disinfection. If a bacterium has a CT requirement of 500 that means we can put 100 milligrams per litre for 5 minutes or 10 milligrams per litre for 50 minutes or say 50 milligrams per litre for 10 minutes.
Ozone and Ultraviolet Light
The ozone and ultraviolet light or ultraviolet irradiation can also be used for disinfection, but these methods of disinfection are relatively less common. The kind of bactericidal effect of chlorine and other disinfection depends on the pH contact time organic content and the fluid temperature. So, all these external factors also control the degree and quality of disinfectant. These are a couple of images. So, a typical chlorine contact tank will look like this. So, this is a buffer system.
 We add chlorine and let the water flow through these baffles and in the residence times if 30 minutes is needed, so in 30 minutes, we’ll ensure the disinfection. UV irradiation basin will look like above where water can be exposed to the UV radiation and that way disinfection can be achieved.
We add chlorine and let the water flow through these baffles and in the residence times if 30 minutes is needed, so in 30 minutes, we’ll ensure the disinfection. UV irradiation basin will look like above where water can be exposed to the UV radiation and that way disinfection can be achieved.
So, conventionally the disinfection is usually last step if it needs to be provided. Of course, if we are going for UV, if you are going for RO also UV is provided before RO and then RO comes the last step but provided like the degree of treatment required if you do not need to go to RO level, so disinfection become of your last treatment.
Nutrient removal process
Tertiary treatment systems employed after secondary treatment systems. So, we call them either tertiary treatment systems or advanced treatment systems.
We discuss about what are the various issues with the existing conventional treatment plants, which teed up to the secondary stage and what are the contaminants that are still there in the water and what kind of targets is set for the advanced treatment system. So, basically what do we need to remove as a tertiary treatment and mostly chemicals used in tertiary system so we will keep focus on this portion.
One of the things was nutrient which is typically not removed that well in conventional treatment systems which usually leads more than like particularly for the domestic sewage, it usually leaves more than 20 milligrams per litre of TK and more than the one milligram per litre of phosphorus.
Why Remove Nutrient?
In order to remove the nutrients, particularly depending on the criteria, as we discussed that for waters which is might be going to use for agricultural purposes, we may not need to bother that much about the nutrient removal, but particularly the one which is going to be disposed in some water body the nutrient pollution could be of point of concern or it could be point of botheration from that perspective.
So, one of the things that is deemed in the tertiary treatment systems or advanced treatment systems is the nutrient removal which we are going to discuss. So, excessive nutrients like nitrogen and phosphorus normally that is what we refer to as nutrients. So, these disrupt the natural environment, the pollution in the surface water bodies, kind of fuels the growth of harmful algae, which is typically known as algal bloom or the more common word eutrophication.
Why aquatic life cannot survive.?
Eutrophication has the ability of completely devastating the aquatic ecosystem. It’s common. So, this eutrophication is caused by the nutrients and when kind of nutrient pollutions are there, it may create the dead zones, which is also called hypoxia. So, this is where aquatic life cannot survive, and they become dead, and this primarily happens because very little or no oxygen.
when this eutrophication happens, the kind of oxygen will be consumed by these algae and furthermore, the rate of oxygen transfer also decreased, because oxygen transferred to this air water interface and since that water, top of the water is covered by this algae by this kind of green material, so, the rate at which oxygen transfer to the water body to the kind of lowers portion of the water body also doesn’t also gets quite slow and as a result, not enough oxygen goes to the water and whatever is available is consumed by the algae.
So that’s why we get very little DO or almost no oxygen in the water, which is covered by the green top. So, that can create the dead zone which is called hypoxia and the aquatic life cannot survive them. water may have higher than acceptable kind of nitrate levels also. So, if somebody is let’s say willing to use this water for some purpose, so the level of nitrate could be higher in these things. Typically, 10 milligrams per litre is permitted but it could be much higher than that which can basically restrict the uses of this for substantial purposes and particularly with for the domestic application or drinking water purposes.
Apart from this algal bloom can create It kind of toxins which can kill the fish and other animals in the water. So, DO depletion or DO decrease is one aspect but it can actually lead to add some toxic ions as well. If these toxics are consumed by the small phase, they will become dead then through this food chain like these are consumed by the bigger fishes and then kind of other animals in the water and from there it can actually lead to even the human food chain.
 Even if algal blooms are kind of not toxic, they can hurt the aquatic life by blocking out the sunlight clogging the fish gills. So, there are a variety of these issues and eutrophication is one of the most sought-after environmental problems across the globe across the world.
Even if algal blooms are kind of not toxic, they can hurt the aquatic life by blocking out the sunlight clogging the fish gills. So, there are a variety of these issues and eutrophication is one of the most sought-after environmental problems across the globe across the world.
The primary reason for eutrophication is the nutrient which is usually disposed off either through agricultural or industrial runoff to the natural water bodies agricultural runoff is one of the major sources of the nutrient because a lot of fertilisers are used in the agricultural activity nitrogen and phosphorus compounds are there in the soil and whenever there is any rainfall happens to through these agricultural runoffs these leads to the nearby water bodies and make them eutrophic.
Removal of Nitrogen and Phosphorus
Whereas the discharge from the like sewage is also one of the point of concerns for the eutrophication point of view, so, nutrient removal that way is an important process in meeting high quality effluent requirement, particularly if it is being targeted for discharge. The removal of nitrogen and phosphorus both can be achieved through chemical or physio-chemical means as well as through biological means.

- Nitrogen removal if we see there are the one of the most common processes nitrification denitrifications, which is a biological method for nitrogen removal.
- Then, there is ammonia stripping, which is physio-chemical method apart from that.
- There are a couple of other methods which are there, but very rarely used like the breakpoint chlorination, where adding the chlorine actually kind of first converts ammonia to the various Chloramines, monochloramine, dichloromethane, trichloromethane and then it can eventually break that chloramine as well to release the nitrogen.
- Ions exchange kind of systems can be used but not from the nutrient removal perspective because it is very rarely used for nutrient removal
- The membrane processes can remove various dissolved ions so, including nitrate, nitrite or ammonia those kinds of things can also be removed by those, but they are high end treatment processes and generally not deployed for removal of the nutrients or nitrogen that way.
we have Biological Phosphorus Removal and chemical Phosphorus Removal methods which we’ll be discussing typical phosphorus concentration in domestic sewage what we get is 4 to 8 milligrams per litre, whereas the nitrogen concentration total nitrogen concentration is 25 to 50 out of which 15 to 35 remains as ammonia and 10 to 20 as organic nitrogen already nitrogen also can be kind of through hydrolysis can be solubilized in the form of ammonia.
The major target for the removal nitrogen removal is the removal of ammonia that’s the one thing in which like nitrogen usually remains dissolved in the state of ammonia until unless it actually is oxidised that way. some nitrite and nitrate could also be there. So, these are the kinds of things that we’ll be discussing.
Nitrogen Removal: Nitrification and Denitrification
The nitrogen removal particularly the nitrification denitrification process is essentially a biological process. The bacteria remove nitrogen from the wastewater in this two-stage process, which is nitrification first and that is followed by the denitrification.
The conversion of ammonia to nitrate through this intermediate nitrite is called nitrification. Whereas, then reduction of this nitrate again through this nitrite eventually to the level of gas phase nitrogen gas is called denitrification.
Nitrification is kind of achieved by the autotrophic group of bacteria whereas denitrification is by the facultative heterotrophic bacteria and since this is the oxidation process usually. This happens in the presence of oxygen whereas, the denitrification is takes place under anoxic conditions usually in the absence of oxygen.
 So, by providing additional aerobic biological treatment these nitrifying bacteria which are mostly present in the water anyway can biologically convert ammonia to the nontoxic nitrate which is the process is called nitrification.
So, by providing additional aerobic biological treatment these nitrifying bacteria which are mostly present in the water anyway can biologically convert ammonia to the nontoxic nitrate which is the process is called nitrification.
However, this needs larger residence time So, we need to give larger retention time if we want to achieve the nitrification process because generally the nitrifier are slow growers so, they grow slow and they act upon slow.
In the conventional treatment system, the residence time that we provide is not good enough for nitrogen so, there is not much of nitrogen removal takes place.
If we intend to basically remove the nitrogen or particularly the ammonia, we can go for a two-step nitrification process. which works with a Nitrosomonas bacteria that converts ammonia or ammonium into the nitrate and then there is a Nitrobacter that converts nitrate to nitrite.
So, this reaction is coupled and kind of proceeds rapidly to the form of nitrates. So, that’s why the nitrite level at any time because as soon as the Nitrosomonas convert ammonia or ammonium into nitrite, the Nitrobacter immediately convert that nitrite to nitrate. So, that’s why the level of nitrite is always very low in the system, which is good also because that’s a toxic species whereas nitrate is far less toxic.
The nitrification process is normally sufficient to remove the toxicity associated with the ammonia in the effluent and so, for several purposes, where we are just bothered about the toxicity, we may not even need to go for any additional step just nitrification is good enough because ammonia has been converted to nitrate and then we release it off or we use at a nutrient because nitrate fertilisers or those kinds of things.
However, in cases where we need to remove the nitrogen completely from the system, we must go for an additional biological process which converts nitrate to nitrogen gas or is basically referred to as denitrification and this is done in an anoxic environment and bacteria use basically the oxygen, which is attached because it’s anoxic environment, there is our anaerobic environment, there is no oxygen present in the system.
So, bacteria use the oxygen attached to the nitrogen in the form of nitrate. This nitrate becomes the terminal electron acceptor in this case, and in the presence of a carbon source in the process of degradation of carbon source they consume that they in order to get that oxygen separate out from the nitrogen they release the nitrogen gas and assimilate or consume the oxygen present in the nitrate.
We have nitrification like the ammonia gets will react with oxygen will form nitrite then this will further react with oxygen will form the nitrate nitrogen. So, overall reaction the ammonia can be converted to the ammonia nitrogen can be converted to nitrate nitrogen, this reflects that this is represented as nitrogen we can remove that also. So, your reaction will still be same way even if we remove.
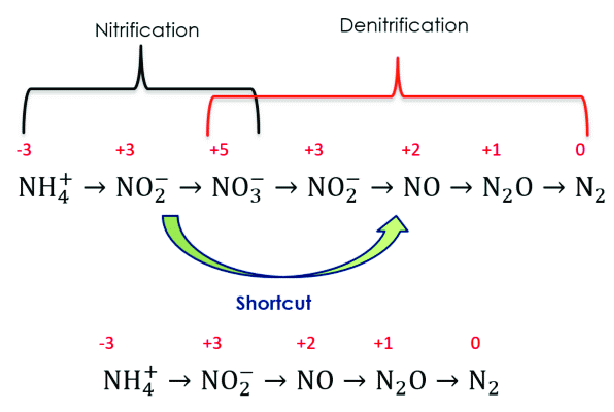 Whereas denitrification process is again this nitrate nitrogen in the presence of carbon source and facultative bacteria it converts to the nitrogen and form some new bacterial cell. So, if we consider the typical like we need a carbon source for this denitrification process, so, if we consider the typical carbon source available in the wastewater in the form of C10 H19 H03N, so then this is the reaction where this nitrate reacts with this and eventually converts the nitrogen converts it to nitrogen.
Whereas denitrification process is again this nitrate nitrogen in the presence of carbon source and facultative bacteria it converts to the nitrogen and form some new bacterial cell. So, if we consider the typical like we need a carbon source for this denitrification process, so, if we consider the typical carbon source available in the wastewater in the form of C10 H19 H03N, so then this is the reaction where this nitrate reacts with this and eventually converts the nitrogen converts it to nitrogen.
Methanol can be another carbon source or acetate can be another carbon source. So, any carbon source that way will be kind of able to through the nitrifier will be able to use that and convert the nitrates into the nitrogen.
Effectiveness
The effective nitrification depends on kind of sufficient oxygen and alkalinity that should be present in the system. So, typically these nitrifier requires 4.57 milligrams of oxygen and 7.14 milligrams of alkalinity as a calcium carbonate for each milligram of nitrate nitrogen form and they you usually yield from 0.06 to 0.2 milligrams per litre of biomass or VSS for each 1 milligrams of nitrate nitrogen, which is being formed in the process of nitrification.
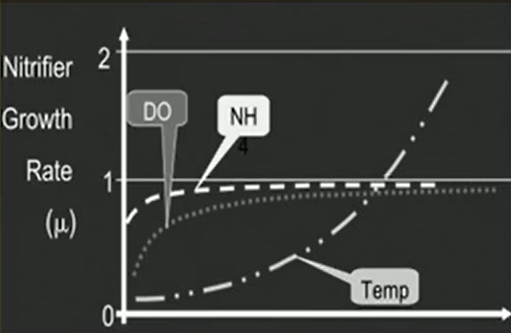 This typically requires a long residence time a low food to microorganism ratio and a high mean cell residence time that way the rate of nitrification can be kind of optimised by controlling these factors and it eventually depends on the temperature and pH as well as temperature increases, the rate increases. we need a kind of optimum pH for this thing as well and DO maintenance is also necessary for the achieving good growth rate of nitrifiers.
This typically requires a long residence time a low food to microorganism ratio and a high mean cell residence time that way the rate of nitrification can be kind of optimised by controlling these factors and it eventually depends on the temperature and pH as well as temperature increases, the rate increases. we need a kind of optimum pH for this thing as well and DO maintenance is also necessary for the achieving good growth rate of nitrifiers.
These nitrifying organisms are present in almost all aerobic processes that way like under favourable conditions, when there is usually the BOD5 to TKN ratio is greater than 5. we can do this in your typical activated sludge process itself. This carbon oxidation and nitrification we can actually do in a single reactor although we need to give larger retention time that is called single stage or combined carbon oxidation and nitrification.
Since this is done in a single state, we call this a single stage process or combined oxidation and nitrification process which can be accomplished in both suspended as well as attached growth systems like activated sludge process, rotating biological contractor Sequencing Batch Reactor, all those things.
The nitrification could also be achieved in a separate reactor, which is called separate stage or two stage process. So, like we have a single stage process we can have go for a two-stage process where the carbon oxidation and nitrification take place in a separate stage and in a separate reactor.
This typically for suspended growth systems it’s the even denitrification design is similar to the design of activated sludge process, there is an attached root system like trickling filter rotating biological contractor or packed bed reactor could also be used for the separate stage nitrification. The separate stage nitrification is usually practised when we have BOD5 to TKN ratio usually less than 3.
The denitrification is accomplished under anaerobic or near anaerobic conditions where the faculty heterotrophic bacteria like Pseudomonas, Micrococcus, achromobactin and bacillus all species eventually they are available in the wastewater typically they are present in the wastewater. So, these convert nitrate to either nitrogen gas by the bacterial metabolism or assimilate nitrogen into their cell mass.
If there is oxygen in the process that actually accepts electron but in the absence of oxygen nitrate becomes the electron acceptor and that way, it is reduced further by accepting electrons.
If the carbon source is present in the wastewater, it is fine if not a supplemental source organic carbon like methanol or acetate those kinds of things can be supplemented for the process for the organic carbon requirements of these anaerobes.
So, these are a couple of these things, so, we can have suspended growth separate stage nitrification process where this is kind of a separate stage nitrification process. Sludge is coming first it is going to the aeration level for BOD removal secondary clarifier is happening and then there is another aeration basin for nitrification and then there is actually a clarifier for nitrate.
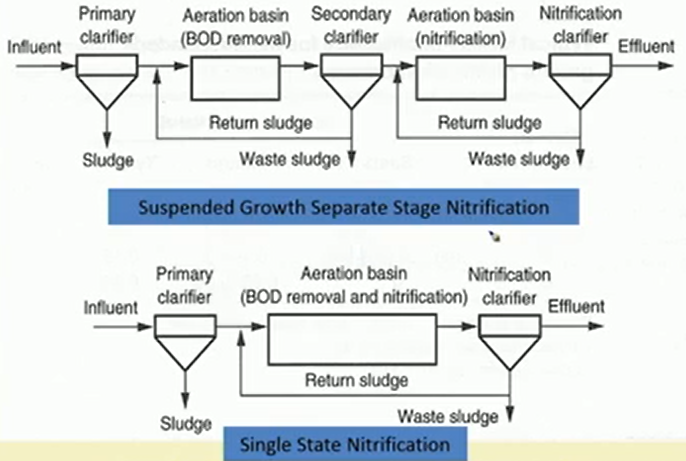 We are actually getting two stages of sludge production as well. This is a typical suspended growth system we can have single stage nitrification as well. So, we get grit of the other part, and the BOD removal and nitrification is done in a single aeration basin only thing is that residence time has to be given a little longer.
We are actually getting two stages of sludge production as well. This is a typical suspended growth system we can have single stage nitrification as well. So, we get grit of the other part, and the BOD removal and nitrification is done in a single aeration basin only thing is that residence time has to be given a little longer.
Pre-anoxic denitrification
There could be various configurations like pre-anoxic denitrification. We put some oxygen before the nitrification So, whatever is there it is converted first then it is again converted to nitrate and then nitrate feet is recycled back to the aerobic system.
 Post-anoxic denitrification
Post-anoxic denitrification
So, that nitrogen is removed. So, that is when we add prior hand, we can have kind of post anoxic system as well. So, where the anoxic join is kept after aerobic So, there is no need of returning nitrate feed over here.
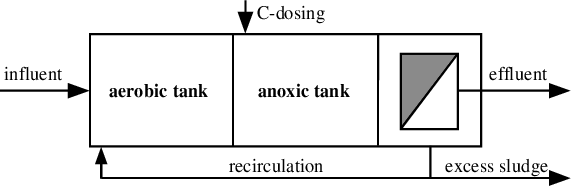 Single sludge system
Single sludge system
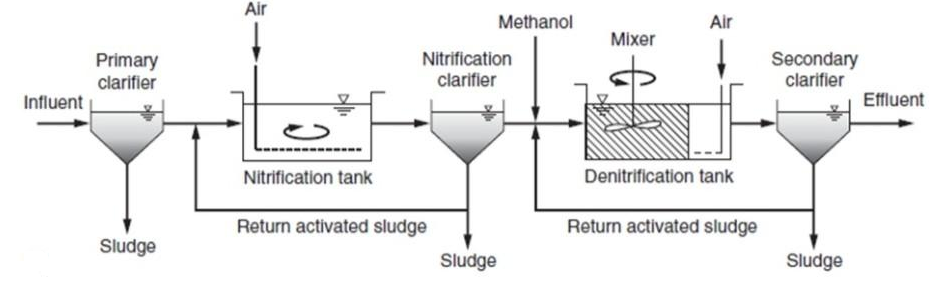
Similarly, we can have single state system or two state sludge production system as just we were seeing So, we can have like nitrification in a separate this thing and denitrification in a separate system that is two stage systems and here since de-nitrification, nitrification is taking place in this thing and then it is going to secondary clarifier.
 Sludge, which is generated contains both kind of sludge whereas, here these nitrifier and Denitrified sludge is kind of separate. So, we have that bit two stage denitrification.
Sludge, which is generated contains both kind of sludge whereas, here these nitrifier and Denitrified sludge is kind of separate. So, we have that bit two stage denitrification.
Two sludge system
 Nitrogen removal: Anammox process
Nitrogen removal: Anammox process
There is another biological nitrogen removal process which is anammox which is kind of a newer term or newer technology. So, this is an anammox is essentially anaerobic ammonium oxidation which we typically call as anammox.
This is discovered in early 90s. Instead of going for the complete process of nitrification and denitrification, it converts the ammonium and nitrite through this partial nitrification into the nitrogen directly. So, we get rid of the conversion into the nitrate and then denitrification of nitrate with the source of acetate all these things are get we get away with this. So, just ammonium and nitrite and are converted to nitrate, it actually the anammox bacteria, which is kind of candidatus, Brocadia and anammox dans.
So, that is used here, and this reaction is something like this. So, ammonia will react with the nitrite and form nitrogen and H2O.
 Advantages
Advantages
- The oxygen addition can be reduced because we are not going for complete nitrification, we are just going for partial nitrification then, these bacteria do not require organic carbon source like methanol and those kinds of things, we are not going for denitrification anyway.
- Production of sludge is also reduced, because larger aeration larger biomass runs, and the reduction of CO2 is also them. CO2 emission is decreased.
So, these are the advantages, whereas it’s a process under development. So, there is kind of a lot of research still needed and there is a high construction cost of these processes. So, these are kind of the demerits of this.
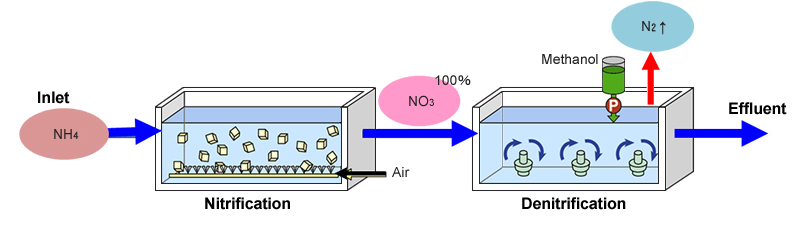
Conventional vs Anammox process
Now, if we compare it with conventional processes, so, in conventional processes, we have ammonia coming through a nitrification basin and then converting into the nitrate which is going to the denitrification releasing nitrogen and effluent and, in the process, it needs some ethanol or some organic carbon source.
 In anammox process, it’s just partial nitrification and then the remaining ammonia and nitrate reacts and kind of forms into that way and little NO3 which goes into the effluent. So, that way is the anammox process is used.
In anammox process, it’s just partial nitrification and then the remaining ammonia and nitrate reacts and kind of forms into that way and little NO3 which goes into the effluent. So, that way is the anammox process is used.
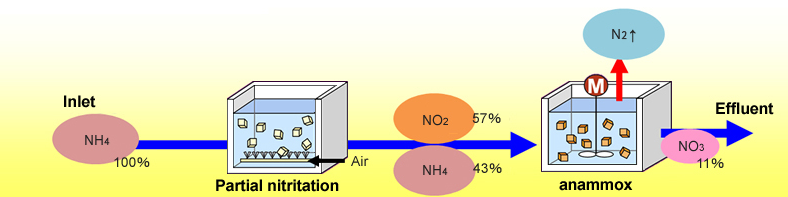 The reactor volume makes become small the oxygen requirement becomes small we do not need any further chemical dosing like methanol and all those kinds of things, starch production is small and kind of optimum temperature this works at 25 to 37 degrees temperature. Control is essential it cannot work in very cold climate that way.
The reactor volume makes become small the oxygen requirement becomes small we do not need any further chemical dosing like methanol and all those kinds of things, starch production is small and kind of optimum temperature this works at 25 to 37 degrees temperature. Control is essential it cannot work in very cold climate that way.
Nitrogen removal: Air or Ammonia stripping
There is air or ammonia stripping which is another method for removal of the nitrogen. The ammonia stripping is again physio-chemical process not a biological process that way so, because there are kind of ammonia present in the system and ammonium ion particularly in such a species that they remain ammonia gaseous. Ammonia and aqueous phase ammonium ion remain together in equilibrium and their equilibrium is controlled by the pH and temperature.
If you can make pH around 11 or 12 So, we see that the maximum portion will be actually in the form of ammonia gas and not in the ammonium ion. So, ammonium ions are something which is dissolved in the water but if it is converted to the ammonia gas can which predominantly exists like kind of an ionised gaseous form. So, that can be kind of then released back.
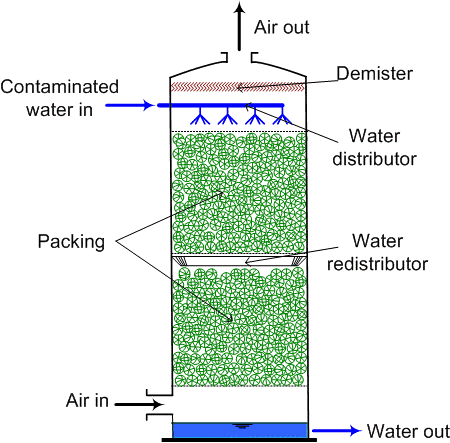 This can be made to escape to the atmosphere if we go for an air stripping process. The idea here is that dissolve ammonium is converted to the gaseous phase and then dispersed in the air this allows kind of transfer of the ammonia from wastewater to the air and pH must be greater than 11 for complete conversion of ammonia, so, around greater than 11 pH at around 20 degrees Celsius, we have 98, 99% of the total nitrogen remaining in the form of ammonia and not the ammonium ion. Ammonium ion is soluble in water that will remain in the water. But if it is converted to ammonia gas, this gas can be basically then stripped off which is done in the ammonia stripping process.
This can be made to escape to the atmosphere if we go for an air stripping process. The idea here is that dissolve ammonium is converted to the gaseous phase and then dispersed in the air this allows kind of transfer of the ammonia from wastewater to the air and pH must be greater than 11 for complete conversion of ammonia, so, around greater than 11 pH at around 20 degrees Celsius, we have 98, 99% of the total nitrogen remaining in the form of ammonia and not the ammonium ion. Ammonium ion is soluble in water that will remain in the water. But if it is converted to ammonia gas, this gas can be basically then stripped off which is done in the ammonia stripping process.
So, typically kind of lime is the most common means for raising the pH lime is added so, that pH is raised there is enough line must be added to precipitate the alkalinity and to kind of increase the chance for the pH adjustment.
The air to water ratio ranges from 2000 to 6000 metre cube of air per metre cube of wastewater which is typically used in the design, and this works well 400 to 10200 milligrams per litre range. There are different kinds of strippers which can be used there are packed bed strippers when there is a packing of this thing.
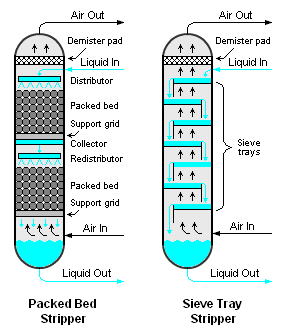 So, we kind of flow liquid from the top and they are from the bottom. So, they pass they interact with each other air gets out liquid gets collected in the bottom or we can have a safe tray. So, where there are trays again liquid moves in So, liquid falls from these trays to each other and then there is an air which kind of through these boards and bubbles moves up.
So, we kind of flow liquid from the top and they are from the bottom. So, they pass they interact with each other air gets out liquid gets collected in the bottom or we can have a safe tray. So, where there are trays again liquid moves in So, liquid falls from these trays to each other and then there is an air which kind of through these boards and bubbles moves up.
Phosphorous removal
Another nutrient that is there in the ETP is phosphorus, which kind of normally secondary treatment can remove 1 to 2 milligrams of phosphorus, so a large excess of phosphorus remains in the outlet of the secondary unit.
There is kind of different forms of phosphorus it could be in the form of ortho phosphates, poly phosphates or originally bond phosphorus. This removal of phosphorus can be achieved through chemical methods or biological methods.
But chemical methods are more reliable and easier to operate that’s why more popular although they are expensive and cause of kind of increased sludge volume, which could increase as high as around 40%.
The alternate Biological Phosphorus Removal methods are also there. They are kind of more environmentally friendly, which are achieved by sequencing and producing the appropriate environmental conditions in the reactor where the bacteria assimilate the phosphorus.
So, from the biological methods, there are specific bacteria which is polyphosphate accumulating organisms (PAOs). So, they have potential to accumulate large quantities of phosphorus within their cell mass.
In their cell structure they can accommodate as high as around 20% of the total mass could be of the phosphorus and that is not in the usual case that is not a specific growth reason, and we have to provide that growth reason.
Enhance Biological Phosphorous Removal (EBPR)
That growth is kind of insured by anaerobic, aerobic sequencing, the COD to phosphorus ratio should be greater than 45 and there must be high phosphorus level in the system. So, for effective removal, these microorganisms PAOs are transferred back and forth between the controlled environment that first forces them to release the phosphorous.
First released the phosphorus from their cell and then once they again introduced in the other system, they adsorb more phosphorus that they wouldn’t normally have and that’s why the accumulation of phosphorus increases.
When this biomass enriched in these bacteria is separated from the treated water the phosphorus level in the water will be decreased because for majority of the Phosphorus has been assimilated into the cell mass.
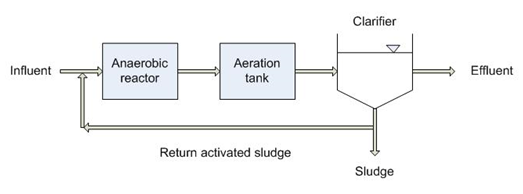 This VFAs is helps in development of these bacteria PAOs and that, because in conventional activity system, they will not develop that much. So, these anoxic environments help in the development of those microorganisms.
This VFAs is helps in development of these bacteria PAOs and that, because in conventional activity system, they will not develop that much. So, these anoxic environments help in the development of those microorganisms.
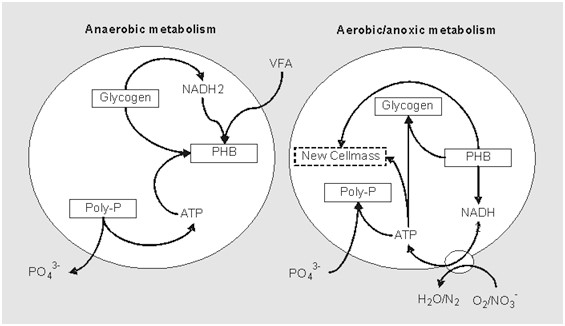 If we see in the anaerobic environment, these PAOs absorb these VFAs which are there in their cells and in the form of kind of internal polymers such as polyhydroxy-butyrate (PHB). So, these VFAs are absorbed as a PHBs in their cell in the anaerobic this thing.
If we see in the anaerobic environment, these PAOs absorb these VFAs which are there in their cells and in the form of kind of internal polymers such as polyhydroxy-butyrate (PHB). So, these VFAs are absorbed as a PHBs in their cell in the anaerobic this thing.
So, in this process phosphates are absorbed from the liquid phase by these PAOs, and that way the concentration of phosphorus decreases, and it actually moves to the cell.
Phosphorous removal: Chemical method
There are a couple of chemical approaches for Phosphorus Removal as well. Chemical approach it is simple chemical precipitation. So, either line-based precipitation or Alum based kind of flocculation and precipitation is what is used.
Lime based precipitation
Lime-based precipitation there is access lime is added in the wastewater and which kind of cause phosphorous to form precipitate with the calcium hydroxide this precipitate then can be flocculated and removed from the water by simple settling. It will react with the phosphate, and it will form this hydroxy loparite which is kind of precipitate So, this is a heavier this will settle down in the system.
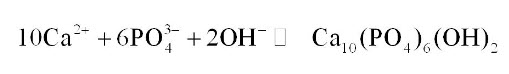 The amount of lime required for this treatment is generally independent of the amount of phosphorus present in the system and will basically depend on the alkalinity because there must be adequate elements present in the system for this reaction to take place or typically pH of the wastewater will be raised because we are adding lime.
The amount of lime required for this treatment is generally independent of the amount of phosphorus present in the system and will basically depend on the alkalinity because there must be adequate elements present in the system for this reaction to take place or typically pH of the wastewater will be raised because we are adding lime.
So, pH will be raised in the range of 10 to 11 with the addition of lime and that way the proper precipitation of phosphorus takes place, which is quite a reliable method, these slots are heavier so, they quickly get settle out.
Precipitation by Alum flocculation
Where aluminium sulphate or alum is added to the wastewater and a precipitate of this aluminium phosphate forms. So, this alum reacts with this thing and a precipitate of this alum phosphate forms this aluminium phosphate is not that dense precipitate and that’s why it needs to be basically some kind of flocculation is needed where we can add a polymer dose or something and it can fluctuate that way.
 Then we can make it settled make it precipitate or we may need to go for the filtration as well because of the presence of the micro flocks of aluminium phosphate in the system. When Alum is added it acts as an acid this reduces the pH of the wastewater by reducing the alkalinity because ultimately will also be reduced alum will react with alkalinity and form alum which also will precipitate so, that excess alum is reduced, but it kind of reduces the alkalinity as well.
Then we can make it settled make it precipitate or we may need to go for the filtration as well because of the presence of the micro flocks of aluminium phosphate in the system. When Alum is added it acts as an acid this reduces the pH of the wastewater by reducing the alkalinity because ultimately will also be reduced alum will react with alkalinity and form alum which also will precipitate so, that excess alum is reduced, but it kind of reduces the alkalinity as well.
So, optimum Phosphorus Removal is generally achieved at a pH range of approximately 6 to 7 apart from these there is kind of Iron salts kind of ferric chloride, or those kinds of coagulant can also be used for the removal of phosphorus. However, these are the more popular ones. So, that way the chemical removal of the phosphorus takes place.
Effluent Treatment Plants Frequently Asked Questions
1) What chemical is most often used in water treatment plants?
The most commonly used chemicals for water treatment process are:
- Algicide
- Chlorine
- Chlorine dioxide
- Muriatic acid
- Soda ash or Sodium bicarbonate
2) What chemicals are used in industrial water treatment?
These substances include sodium hydroxide, hydrated lime, sulfuric acid, and hydrochloric acid as examples. Coagulation agents and flocculants are additional typical treatment chemicals. These substances aid in making wastewater contaminants adhere to one another, making it simpler to eliminate them from water.
3) What is the process of removal of nitrogen?
Nitrification and DE nitrification are the two processes in biological treatment that remove nitrogen. Total ammonia, which includes free ammonia and unionized ammonia, is converted to nitrate in this process by nitrifiers such as ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB).
4) What are the advantages of having both ozone and UV light in a water treatment?
The “power of three” for improved disinfection and oxidation efficacy brought about by the UV/ozone combination is related to the production of hydroxyl radicals.
Ozone and UV are two widely used techniques for disinfecting water and wastewater in the commercial and municipal sectors. Each one is capable of rendering viruses inactive. For water and wastewater disinfection as well as many industrial uses, UV has been shown to be an affordable alternative.
5) Which is the most commonly used disinfectant?
Chemical Disinfectants
- Alcohol
- Chlorine and chlorine compounds
- Formaldehyde
- Glutaraldehyde
- Hydrogen peroxide
- Iodophors
- Ortho-phthalaldehyde (OPA)
- Peracetic acid